RFP No. 212F for Endangered Species Research Projects for the Prairie Chub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Carmine Shiner (Notropis Percobromus) in Canada
COSEWIC Assessment and Update Status Report on the Carmine Shiner Notropis percobromus in Canada THREATENED 2006 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2006. COSEWIC assessment and update status report on the carmine shiner Notropis percobromus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 29 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Previous reports COSEWIC 2001. COSEWIC assessment and status report on the carmine shiner Notropis percobromus and rosyface shiner Notropis rubellus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. v + 17 pp. Houston, J. 1994. COSEWIC status report on the rosyface shiner Notropis rubellus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-17 pp. Production note: COSEWIC would like to acknowledge D.B. Stewart for writing the update status report on the carmine shiner Notropis percobromus in Canada, prepared under contract with Environment Canada, overseen and edited by Robert Campbell, Co-chair, COSEWIC Freshwater Fishes Species Specialist Subcommittee. In 1994 and again in 2001, COSEWIC assessed minnows belonging to the rosyface shiner species complex, including those in Manitoba, as rosyface shiner (Notropis rubellus). For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Évaluation et Rapport de situation du COSEPAC sur la tête carminée (Notropis percobromus) au Canada – Mise à jour. -

Notropis Girardi) and Peppered Chub (Macrhybopsis Tetranema)
Arkansas River Shiner and Peppered Chub SSA, October 2018 Species Status Assessment Report for the Arkansas River Shiner (Notropis girardi) and Peppered Chub (Macrhybopsis tetranema) Arkansas River shiner (bottom left) and peppered chub (top right - two fish) (Photo credit U.S. Fish and Wildlife Service) Arkansas River Shiner and Peppered Chub SSA, October 2018 Version 1.0a October 2018 U.S. Fish and Wildlife Service Region 2 Albuquerque, NM This document was prepared by Angela Anders, Jennifer Smith-Castro, Peter Burck (U.S. Fish and Wildlife Service (USFWS) – Southwest Regional Office) Robert Allen, Debra Bills, Omar Bocanegra, Sean Edwards, Valerie Morgan (USFWS –Arlington, Texas Field Office), Ken Collins, Patricia Echo-Hawk, Daniel Fenner, Jonathan Fisher, Laurence Levesque, Jonna Polk (USFWS – Oklahoma Field Office), Stephen Davenport (USFWS – New Mexico Fish and Wildlife Conservation Office), Mark Horner, Susan Millsap (USFWS – New Mexico Field Office), Jonathan JaKa (USFWS – Headquarters), Jason Luginbill, and Vernon Tabor (Kansas Field Office). Suggested reference: U.S. Fish and Wildlife Service. 2018. Species status assessment report for the Arkansas River shiner (Notropis girardi) and peppered chub (Macrhybopsis tetranema), version 1.0, with appendices. October 2018. Albuquerque, NM. 172 pp. Arkansas River Shiner and Peppered Chub SSA, October 2018 EXECUTIVE SUMMARY ES.1 INTRODUCTION (CHAPTER 1) The Arkansas River shiner (Notropis girardi) and peppered chub (Macrhybopsis tetranema) are restricted primarily to the contiguous river segments of the South Canadian River basin spanning eastern New Mexico downstream to eastern Oklahoma (although the peppered chub is less widespread). Both species have experienced substantial declines in distribution and abundance due to habitat destruction and modification from stream dewatering or depletion from diversion of surface water and groundwater pumping, construction of impoundments, and water quality degradation. -

From the Western Mosquitofish, Gambusia Affinis (Cyprinodontiformes: Poeciliidae): New Distributional Records for Arkansas, Kansas and Oklahoma
42 Salsuginus seculus (Monogenoidea: Dactylogyrida: Ancyrocephalidae) from the Western Mosquitofish, Gambusia affinis (Cyprinodontiformes: Poeciliidae): New distributional records for Arkansas, Kansas and Oklahoma Chris T. McAllister Science and Mathematics Division, Eastern Oklahoma State College, Idabel, OK 74745 Donald G. Cloutman P. O. Box 197, Burdett, KS 67523 Henry W. Robison Department of Biology, Southern Arkansas University, Magnolia, AR 71754 Studies on fish monogeneans in Oklahoma and S. fundulus (Mizelle) Murith and Beverley- are relatively uncommon (Seamster 1937, 1938, Burton in Northern Studfish,Fundulus catenatus 1960; Mizelle 1938; Monaco and Mizelle 1955; (McAllister et al. 2015, 2016). In Kansas, a McDaniel 1963; McDaniel and Bailey 1966; single species, S. thalkeni Janovy, Ruhnke, and Wheeler and Beverley-Burton 1989) with little Wheeler (syn. S. fundulus) has been reported or no published work in the past two decades from Northern Plains Killifish,Fundulus kansae or more. Members of the ancyrocephalid (see Janovy et al. 1989). Here, we report genus Salsuginus (Beverley-Burton) Murith new distributional records for a species of and Beverley-Burton have been reported Salsuginus in Arkansas, Kansas and Oklahoma. from various fundulid fishes including those from Alabama, Arkansas, Illinois, Kentucky, During June 1983 (Kansas only) and again Nebraska, New York, Tennessee, and Texas, between April 2014 and September 2015, and Newfoundland and Ontario, Canada, 36 Western Mosquitofish, Gambusia affinis and the Bahama Islands; additionally, two were collected by dipnet, seine (3.7 m, 1.6 species have been reported from the Western mm mesh) or backpack electrofisher from Big Mosquitofish, Gambusia affinis (Poeciliidae) Spring at Spring Mill, Independence County, from California, Louisiana, and Texas, and Arkansas (n = 4; 35.828152°N, 91.724273°W), the Bahama Islands (see Hoffman 1999). -

The Native Fish Fauna of Major Drainages East of The
THE NATIVE FISH FAUNA OF MAJOR DRAINAGES EAST OF THE CONTINENTAL DIVIDE IN NEW MEXICO A Thesis Presented to the Graduate Faculty of Biology Eastern New Mexico University In Partial Fulfillment of the Requirements fdr -the7Degree: Master of Science in Biology by Michael D. Hatch December 1984 TABLE OF CONTENTS Page Introduction Study Area Procedures Results and Discussion Summary Acknowledgements Literature Cited Appendices Abstract INTRODUCTION r (t. The earliest impression of New Mexico's native fish fauna =Ems during the 1850's from naturalists attached to various government survey parties. Without the collections from these and other early surveys, the record of the native fish fauna would be severely deficient because, since that time, some 1 4 native species - or subspecies of fish have become extirpated and the ranges of an additionial 22 native species or subspecies have become severly re- stricted. Since the late Miocene, physiographical changes of drainages have linked New Mexico, to varying degrees, with contemporary ichthyofaunal elements or their progenitors from the Rocky Mountains, the Great Plains, the Chihuahuan Desert, the Mexican Plateau, the Sonoran Desert and the Great Basin. Immigra- tion from these areas contributed to the diversity of the state's native ichthyofauna. Over the millinea, the fate of these fishes waxed and waned in ell 4, response to the changing physical and _chenaca-l-conditions of the surrounding environment. Ultimately, one of the most diverse fish faunas of any of the interior southwestern states developed. Fourteen families comprising 67 species of fish are believed to have occupied New Mexico's waters historically, with strikingly different faunas evolving east and west of the Continental Divide. -

Historic Document – Content May Not Reflect Current Scientific Research, Policies Or Practices
U.S. Department of Agriculture Animal and Plant Health Inspection Service Wildlife Services Historic document – Content may not reflect current scientific research, policies or practices. COOPERATIVE FISHERY UNIT REPORT FOR THE PERIOD JANUARY 1968 THROUGH JUNE 1969 TRAIN ING UNITED STATES DEPARTMENT OF THE INTERIOR FISH AND WILDLIFE SERVICE BUREAU OF SPORT FISHERIES AND WILDLIFE DIVISION OF FISHERY SERVICES RESOURCE PUBLICATION 80 E RESl=AP-CH CENTEI"' {lBRARV WILDUF - · ' ~ ~ilill)lll~l~~ll I: S/'~~ P~ .'"'.' ~::'~ ~c·~~i E 90018774 OUNER, COL01\KUO t02 As the Nation's principal conservation agency, the Depart- ment of the Interior has basic responsibilitie s for water, fish, wildlife, mineral, land, park, and recreational re- sources. Indian and Territorial affairs are ocher major concerns of America's "Department of Natural Resources." The Department works co assure the wisest choice in managing all our resources so each will ma lee its full contribution to a better United States -- now and in the future. Cover drawing by Craig P. Phillips, National Fisheries Center and Aquarium, Washington, D.C. For sole by tho Superlnumdont ol Docwncnta, U.S. Oovernmont Prlnll11& omce Wo.shln&ton, D.C. 20402 • Price 76 oont.s UNITED STATES DEPARTMENT OF THE INTERIOR , WALTER J . HICKEL , SECRETARY Leslie L. Glasgow, Assistant Secretary for Fish and Wi ldlif e, Parks, and Marine Resources Fish and Wildlife Service, Charles H. Meacham , Corrunissioner Bureau of Sport Fisheries and Wildlife, John S. Gottschalk, Direct or COOPERATIVE FISHERY UNIT REPORT for the Period January 1968 through June 1969 A Nation-wide Cooperative Program of Training, Investigation and Application by the Bureau of Sport Fisheries and Wildlife, State Game and Fi s h Departments, and Colleges and Universities . -
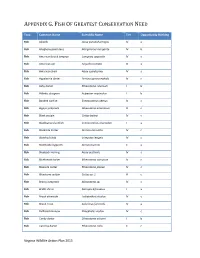
Fish of Greatest Conservation Need
APPENDIX G. FISH OF GREATEST CONSERVATION NEED Taxa Common Name Scientific Name Tier Opportunity Ranking Fish Alewife Alosa pseudoharengus IV a Fish Allegheny pearl dace Margariscus margarita IV b Fish American brook lamprey Lampetra appendix IV c Fish American eel Anguilla rostrata III a Fish American shad Alosa sapidissima IV a Fish Appalachia darter Percina gymnocephala IV c Fish Ashy darter Etheostoma cinereum I b Fish Atlantic sturgeon Acipenser oxyrinchus I b Fish Banded sunfish Enneacanthus obesus IV c Fish Bigeye jumprock Moxostoma ariommum III c Fish Black sculpin Cottus baileyi IV c Fish Blackbanded sunfish Enneacanthus chaetodon I a Fish Blackside darter Percina maculata IV c Fish Blotched chub Erimystax insignis IV c Fish Blotchside logperch Percina burtoni II a Fish Blueback Herring Alosa aestivalis IV a Fish Bluebreast darter Etheostoma camurum IV c Fish Blueside darter Etheostoma jessiae IV c Fish Bluestone sculpin Cottus sp. 1 III c Fish Brassy Jumprock Moxostoma sp. IV c Fish Bridle shiner Notropis bifrenatus I a Fish Brook silverside Labidesthes sicculus IV c Fish Brook Trout Salvelinus fontinalis IV a Fish Bullhead minnow Pimephales vigilax IV c Fish Candy darter Etheostoma osburni I b Fish Carolina darter Etheostoma collis II c Virginia Wildlife Action Plan 2015 APPENDIX G. FISH OF GREATEST CONSERVATION NEED Fish Carolina fantail darter Etheostoma brevispinum IV c Fish Channel darter Percina copelandi III c Fish Clinch dace Chrosomus sp. cf. saylori I a Fish Clinch sculpin Cottus sp. 4 III c Fish Dusky darter Percina sciera IV c Fish Duskytail darter Etheostoma percnurum I a Fish Emerald shiner Notropis atherinoides IV c Fish Fatlips minnow Phenacobius crassilabrum II c Fish Freshwater drum Aplodinotus grunniens III c Fish Golden Darter Etheostoma denoncourti II b Fish Greenfin darter Etheostoma chlorobranchium I b Fish Highback chub Hybopsis hypsinotus IV c Fish Highfin Shiner Notropis altipinnis IV c Fish Holston sculpin Cottus sp. -

Northern Plains Killifish - Fundulus Kansae Abundance: Common Status: NSS3 (Bb) Natureserve: G5 S5 Population Status: Stable
Northern plains Killifish - Fundulus kansae Abundance: Common Status: NSS3 (Bb) NatureServe: G5 S5 Population Status: Stable. Distribution and abundance appears stable over last decade. Limiting Factor: Habitat: severe due to limited availability of shallow, sandy habitats. Comment: Changed from NSS4 to NSS3 in 2017 due to recent surveys indicating that the distribution of this species is more restricted than previously believed within native range. Introduction The northern plains killifish is native to the Great Plains region of central North America, where it ranges from southeast Montana, South Dakota, and Missouri south to Texas (Rahel and Thel 2004). Populations have been introduced in Colorado, Utah, Arizona, New Mexico, Montana, Wyoming, Texas, and South Dakota (Rahel and Thel 2004). In Wyoming, northern plains killifish are indigenous to the North Platte and South Platte drainages, but are also found outside their range in the Big Horn and Cheyenne river drainages (Baxter and Stone 1995), possibly introduced by bait fisherman (Baxter and Simon 1970). Baxter and Simon (1970) reported no findings of northern plains killifish within the Powder River basin during 1964 sampling. Patton (1997) was the first to record the presence of this species in the Powder River system. Given this, they are likely not native to the Powder River drainage. Northern plains killifish are typically carnivorous, consuming a variety of insects and other aquatic invertebrates, and occasionally eat plant material and diatoms (Minckley and Klaassen 1969; Pflieger 1997; Rahel and Thel 2004). They feed at the surface, in the water column, and from the bottom substrate (Baxter and Stone 1995). Spawning takes place from May to August and may be stimulated by temperature and flow cues (Pflieger 1997; Rahel and Thel 2004). -
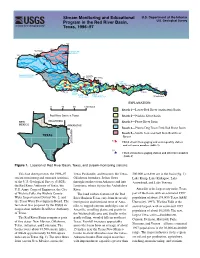
Stream Monitoring and Educational Program in the Red River Basin
Stream Monitoring and Educational U.S. Department of the Interior Program in the Red River Basin, U.S. Geological Survey Texas, 1996–97 100 o 101 o 5 AMARILLO NORTH FORK 102 o RED RIVER 103 o A S LT 35o F ORK RED R IV ER 1 4 2 PRAIRIE DOG TOWN PEASE 3 99 o WICHITA FORK RED RIVER 7 FALLS CHARLIE 6 RIVE R o o 34 W 8 98 9 I R o LAKE CHIT 21 ED 97 A . TEXOMA o VE o 10 11 R 25 96 RI R 95 16 19 18 20 DENISON 17 28 14 15 23 24 27 29 22 26 30 12,13 LAKE PARIS KEMP LAKE LAKE KICKAPOO ARROWHEAD TEXARKANA EXPLANATION 0 40 80 120 MILES Reach 1—Lower Red River (mainstem) Basin Red River Basin in Texas Reach 2—Wichita River Basin NEW OKLAHOMA Reach 3—Pease River Basin MEXICO ARKANSAS Reach 4—Prairie Dog Town Fork Red River Basin Reach 5—North Fork and Salt Fork Red River TEXAS Basins 12 LOUISIANA USGS streamflow-gaging and water-quality station and reference number (table 1) 22 USGS streamflow-gaging station and reference number (table 1) Figure 1. Location of Red River Basin, Texas, and stream-monitoring stations. This fact sheet presents the 1996–97 Texas Panhandle, and becomes the Texas- 200,000 acre-feet are in the basin (fig. 1): stream monitoring and outreach activities Oklahoma boundary. It then flows Lake Kemp, Lake Kickapoo, Lake of the U.S. Geological Survey (USGS), through southwestern Arkansas and into Arrowhead, and Lake Texoma. -

Assessing Biological Integrity in Running Waters a Method and Its Rationale
Assessing Biological Integrity in Running Waters A Method and Its Rationale James R. Karr Kurt D. Fausch Paul L. Angermeier Philip R. Yant Isaac J. Schlosser Jordan Creek ---------------- ] Excellent !:: ~~~~~~~~~;~~;~~ ~ :: ,. JPoor --------------- 111 1C tE 2A 28 20 3A SO 3E 4A 48 4C 40 4E Station Illinois Natural History Survey Special Publication 5 September 1986 Printed by authority of the State of Illinois Illinois Natural History Survey 172 Natural Resources Building 607 East Peabody Drive Champaign, Illinois 61820 The Illinois Natural History Survey is pleased to publish this report and make it available to a wide variety of potential users. The Survey endorses the concepts from which the Index of Biotic Integrity was developed but cautions, as the authors are careful to indicate, that details must be tailored to lit the geographic region in which the Index is to be used. Glen C. Sanderson, Chair, Publications Committee, Illinois Natural History Survey R. Weldon Larimore of the Illinois Natural History Survey took the cover photos, which show two reaches ofJordan Creek in east-central Illinois-an undisturbed site and a site that shows the effects of grazing and agricultural activity. Current affiliations of the authors are listed below: James R. Karr, Deputy Director, Smithsonian Tropical Research Institute, Balboa, Panama Kurt D. Fausch, Department of Fishery and Wildlife Biology, Colorado State University, Fort Collins Paul L. Angermeier, Department of Fisheries and Wildlife Sciences, Virginia Polytechnic Institute and State University, Blacksburg Philip R. Yant, Museum of Zoology, University of Michigan, Ann Arbor Isaac J. Schlosser, Department of Biology, University of North Dakota, Grand Forks VDP-1-3M-9-86 ISSN 0888-9546 Assessing Biological Integrity in Running Waters A Method and Its Rationale James R. -

National Register Nomination File
NPS Form 10-900 OMB No. 1024-0018 United States Department of the Interior National Park Service National Register of Historic Places Registration Form 1. Name of Property Historic Name: SH 203 (Old SH 52) Bridge at Salt Fork of the Red River Other name/site number: State Highway 52 Bridge at Salt Fork of the Red River Name of related multiple property listing: Historic Road Infrastructure of Texas 2. Location Street & number: Texas State Highway 203 at Salt Fork of the Red River City or town: Wellington State: Texas County: Collingsworth Not for publication: Vicinity: 3. State/Federal Agency Certification As the designated authority under the National Historic Preservation Act, as amended, I hereby certify that this nomination request for determination of eligibility meets the documentation standards for registering properties in the National Register of Historic Places and meets the procedural and professional requirements set forth in 36 CFR Part 60. In my opinion, the property meets does not meet the National Register criteria. I recommend that this property be considered significant at the following levels of significance: national statewide local Applicable National Register Criteria: A B C D State Historic Preservation Officer ___________________________ Signature of certifying official / Title Date Texas Historical Commission State or Federal agency / bureau or Tribal Government In my opinion, the property meets does not meet the National Register criteria. _______________________________________________________________________ ___________________________ Signature of commenting or other official Date ____________________________________________________________ State or Federal agency / bureau or Tribal Government 4. National Park Service Certification I hereby certify that the property is: ___ entered in the National Register ___ determined eligible for the National Register ___ determined not eligible for the National Register. -

Great Bowls of Fire Get Bragging Rights and a Handsome Keeping Your Community Strong
THE CLARENDON 11.26.2015 The Texas Panhandle’s First Newspaper. Established 1878. Enterprise THE CLARENDON NEWS & THE DONLEY COUNTY LEADER www.ClarendonLive.com Single 00 Courthouse lighting, chili cookoff this Saturday Copy $1 The Christmas season will start endonLive.com. community. Chili Challenge with a chance to win plaque, and the ballot of one lucky in a big way in Clarendon this week- Small Business Saturday began Saturday’s highlight will be the $250 in Clarendon Cash. taster will receive $250 in Clarendon THIS WEEK end with merchant sales, the light- in 2010 when American Express second annual Courthouse Light- Chili Challenge ballots and a Cash from the Chamber. ing of the Courthouse, and the third founded it to help small businesses ing Celebration, beginning at 5 p.m. list of participating merchants will Also on Saturday night, the 2 Shopping at small annual Chili Challenge. get more exposure during one of the with an appearance by Santa Claus be available for $5 each from the VFW Ladies Auxiliary will hold a businesses can make a big Local merchants are ready to biggest shopping weekends of the and caroling featuring local chil- Clarendon Visitor Center at Mulkey soup dinner at the Clarendon Lions impact this holiday season. help you find something for every- year. dren’s groups. The formal lighting Theatre immediately following the Hall for $5 per person featuring 4 Peggy recalls past holiday one on your Christmas list with their According to small business will be at 6 p.m. Courthouse Lighting. Chili tasters homemade chicken noodle or potato cooking disasters. -

Santa Fe National Forest
Chapter 1: Introduction In Ecological and Biological Diversity of National Forests in Region 3 Bruce Vander Lee, Ruth Smith, and Joanna Bate The Nature Conservancy EXECUTIVE SUMMARY We summarized existing regional-scale biological and ecological assessment information from Arizona and New Mexico for use in the development of Forest Plans for the eleven National Forests in USDA Forest Service Region 3 (Region 3). Under the current Planning Rule, Forest Plans are to be strategic documents focusing on ecological, economic, and social sustainability. In addition, Region 3 has identified restoration of the functionality of fire-adapted systems as a central priority to address forest health issues. Assessments were selected for inclusion in this report based on (1) relevance to Forest Planning needs with emphasis on the need to address ecosystem diversity and ecological sustainability, (2) suitability to address restoration of Region 3’s major vegetation systems, and (3) suitability to address ecological conditions at regional scales. We identified five assessments that addressed the distribution and current condition of ecological and biological diversity within Region 3. We summarized each of these assessments to highlight important ecological resources that exist on National Forests in Arizona and New Mexico: • Extent and distribution of potential natural vegetation types in Arizona and New Mexico • Distribution and condition of low-elevation grasslands in Arizona • Distribution of stream reaches with native fish occurrences in Arizona • Species richness and conservation status attributes for all species on National Forests in Arizona and New Mexico • Identification of priority areas for biodiversity conservation from Ecoregional Assessments from Arizona and New Mexico Analyses of available assessments were completed across all management jurisdictions for Arizona and New Mexico, providing a regional context to illustrate the biological and ecological importance of National Forests in Region 3.