Cotingidae, Aves) with a Comparative Evolutionary Analysis of Breeding System and Plumage Dimorphism and a Revised Phylogenetic Classification ⇑ Jacob S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acari: Syringophilidae: Picobiinae) Associated with Woodcreeper Birds (Passeriformes: Dendrocolaptidae)
Zootaxa 3406: 59–66 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2012 · Magnolia Press ISSN 1175-5334 (online edition) New picobiin mites (Acari: Syringophilidae: Picobiinae) associated with woodcreeper birds (Passeriformes: Dendrocolaptidae) MACIEJ SKORACKI1, 3& PIOTR SOLARCZYK2 1Department of Animal Morphology, Faculty of Biology, Adam Mickiewicz University, Umultowska 89, 61–614 Poznan, Poland 2Department of Biology and Medical Parasitology, Poznan University of Medical Sciences, Faculty of Medicine I, 10 Fredry Street, Poznan, Poland 3Corresponding author. E-mail: [email protected] Abstract Two new species belonging to the genus Rafapicobia Skoracki, 2011 (Syringophilidae: Picobiinae) collected from birds of the family Dendrocolaptidae are described: 1) Rafapicobia dendrocolaptesi sp. nov. from Dendrocolaptes platyrostris Spix (type host) in Paraguay and from D. picumnus Lichtenstein in Argentina; 2) Rafapicobia lepidocolaptesi sp. nov. from Lepidocolaptes affinis (Lafresnaye) (type host) in Ecuador and Venezuela and from L. souleyetii (Des Murs) in Co- lombia. Syringophilid mites are recorded from woodcreepers for the first time. Key words: Acari, Syringophilidae, Picobiinae, quill mites, ectoparasites, birds, Dendrocolaptidae Introduction The subfamily Picobiinae Johnson and Kethley (Acariformes: Cheyletoidea: Syringophilidae) is potentially taxo- nomically diverse group of the syringophilid mites parasitising birds. All members of this subfamily occupy exclu- sively quills of body -

A Comprehensive Multilocus Phylogeny of the Neotropical Cotingas
Molecular Phylogenetics and Evolution 81 (2014) 120–136 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev A comprehensive multilocus phylogeny of the Neotropical cotingas (Cotingidae, Aves) with a comparative evolutionary analysis of breeding system and plumage dimorphism and a revised phylogenetic classification ⇑ Jacob S. Berv 1, Richard O. Prum Department of Ecology and Evolutionary Biology and Peabody Museum of Natural History, Yale University, P.O. Box 208105, New Haven, CT 06520, USA article info abstract Article history: The Neotropical cotingas (Cotingidae: Aves) are a group of passerine birds that are characterized by Received 18 April 2014 extreme diversity in morphology, ecology, breeding system, and behavior. Here, we present a compre- Revised 24 July 2014 hensive phylogeny of the Neotropical cotingas based on six nuclear and mitochondrial loci (7500 bp) Accepted 6 September 2014 for a sample of 61 cotinga species in all 25 genera, and 22 species of suboscine outgroups. Our taxon sam- Available online 16 September 2014 ple more than doubles the number of cotinga species studied in previous analyses, and allows us to test the monophyly of the cotingas as well as their intrageneric relationships with high resolution. We ana- Keywords: lyze our genetic data using a Bayesian species tree method, and concatenated Bayesian and maximum Phylogenetics likelihood methods, and present a highly supported phylogenetic hypothesis. We confirm the monophyly Bayesian inference Species-tree of the cotingas, and present the first phylogenetic evidence for the relationships of Phibalura flavirostris as Sexual selection the sister group to Ampelion and Doliornis, and the paraphyly of Lipaugus with respect to Tijuca. -

Systematic Relationships and Biogeography of the Tracheophone Suboscines (Aves: Passeriformes)
MOLECULAR PHYLOGENETICS AND EVOLUTION Molecular Phylogenetics and Evolution 23 (2002) 499–512 www.academicpress.com Systematic relationships and biogeography of the tracheophone suboscines (Aves: Passeriformes) Martin Irestedt,a,b,* Jon Fjeldsaa,c Ulf S. Johansson,a,b and Per G.P. Ericsona a Department of Vertebrate Zoology and Molecular Systematics Laboratory, Swedish Museum of Natural History, P.O. Box 50007, SE-104 05 Stockholm, Sweden b Department of Zoology, University of Stockholm, SE-106 91 Stockholm, Sweden c Zoological Museum, University of Copenhagen, Copenhagen, Denmark Received 29 August 2001; received in revised form 17 January 2002 Abstract Based on their highly specialized ‘‘tracheophone’’ syrinx, the avian families Furnariidae (ovenbirds), Dendrocolaptidae (woodcreepers), Formicariidae (ground antbirds), Thamnophilidae (typical antbirds), Rhinocryptidae (tapaculos), and Conop- ophagidae (gnateaters) have long been recognized to constitute a monophyletic group of suboscine passerines. However, the monophyly of these families have been contested and their interrelationships are poorly understood, and this constrains the pos- sibilities for interpreting adaptive tendencies in this very diverse group. In this study we present a higher-level phylogeny and classification for the tracheophone birds based on phylogenetic analyses of sequence data obtained from 32 ingroup taxa. Both mitochondrial (cytochrome b) and nuclear genes (c-myc, RAG-1, and myoglobin) have been sequenced, and more than 3000 bp were subjected to parsimony and maximum-likelihood analyses. The phylogenetic signals in the mitochondrial and nuclear genes were compared and found to be very similar. The results from the analysis of the combined dataset (all genes, but with transitions at third codon positions in the cytochrome b excluded) partly corroborate previous phylogenetic hypotheses, but several novel arrangements were also suggested. -

Trip Report February 25 – March 9, 2018 | Written by Keith Hansen
Guyana: Unspoiled Wilderness | Trip Report February 25 – March 9, 2018 | Written by Keith Hansen With Local Guide Leon Moore, Keith Hansen, and participants Kirk, Clifton, Margaret, Karl, John, Paul, Goly, David, and Dottie Naturalist Journeys, LLC / Caligo Ventures PO Box 16545 Portal, AZ 85632 PH: 520.558.1146 / 800.426.7781 Fax 650.471.7667 naturalistjourneys.com / caligo.com [email protected] / [email protected] Guyana: Unspoiled Wilderness | Trip Report February 25 – March 9, 2018 | Written by Keith Hansen Before my accounting of the Naturalist Journeys 2018 tour to Guyana, I want to personally thank the many, many people who helped to create this incredible experience. First, my heartfelt thanks, goes to Peg Abbott and the ENTIRE staff at Naturalist Journeys and to everyone on the ground in Guyana. From the caring and efficient hotel and lodge staffs to the tireless and gifted food preparers, to the brave taxi drivers and skilled boatmen, to our faithful crew of able and experienced drivers, every expert local- guide and then finally, Leon. On so many levels, this trip just could not have been what it was without his steady, patient, and knowledgeable guidance. We were truly in good hands. To the nine fellow participants on this adventure, I want to say to each and all of you that it was my pleasure to share this trip with you. Your involvement, powers of observation, sharp eyes, and quick directions all helped to broaden our horizon, thereby increasing the enjoyment and expanding the richness of the collective experience. Thank you. Sun., Feb. 25 Arrival in Georgetown | Cara Lodge While some arrived the day before, our group of nine participants gathered today for the official start of our Naturalist Journeys’ tour at the Cara Hotel in Georgetown, Guyana. -

Nest Architecture and Placement of Three Manakin Species in Lowland Ecuador José R
Cotinga29-080304.qxp 3/4/2008 10:42 AM Page 57 Cotinga 29 Nest architecture and placement of three manakin species in lowland Ecuador José R. Hidalgo, Thomas B. Ryder, Wendy P. Tori, Renata Durães, John G. Blake and Bette A. Loiselle Received 14 July 2007; final revision accepted 24 September 2007 Cotinga 29 (2008): 57–61 El conocimiento sobre los hábitat de reproducción de muchas especies tropicales es limitado. En particular, la biología de anidación de varias especies de la familia Pipridae, conocida por sus carac- terísticos despliegues de machos en asambleas de cortejo, es poco conocida. Aquí proporcionamos descripciones y comparamos la arquitectura de nidos y lugares de anidación utilizados por tres especies de saltarines: Saltarín Cola de Alambre Pipra filicauda,Saltarín Coroniblanco P. pipra y Saltarín Coroniazul Lepidothrix coronata,presentes en la Amazonia baja del Ecuador.Encontramos 76 nidos de P. filicauda,13 nidos de P. pipra y 41 nidos de L. coronata.Los resultados indican que P. filicauda y L. coronata usan hábitat similares para anidar (flancos o crestas de quebradas pequeñas), mientras que P. pipra tiende a anidar en sitios relativamente abiertos rodeados por sotobosque más denso. Las tres especies construyen nidos pequeños, poco profundos, los cuales, a pesar de ser similares, son distinguibles debido a sus componentes estructurales y características de ubicación. Este estudio contribuye a nuestro conocimiento sobre los hábitos de anidación de la avifauna tropical. Manakins (Pipridae) are widespread throughout Guiana16,and L. coronata from Central America13). warm and humid regions of Central and South There is no published information, however, on the America4,10,14,but reach their greatest diversity in nesting biology of these three species in Ecuador lowland Amazonia where up to eight species may and, due to observed geographic variation, such occur in sympatry1,4,14.Manakins are small, data are valuable. -

Tinamiformes – Falconiformes
LIST OF THE 2,008 BIRD SPECIES (WITH SCIENTIFIC AND ENGLISH NAMES) KNOWN FROM THE A.O.U. CHECK-LIST AREA. Notes: "(A)" = accidental/casualin A.O.U. area; "(H)" -- recordedin A.O.U. area only from Hawaii; "(I)" = introducedinto A.O.U. area; "(N)" = has not bred in A.O.U. area but occursregularly as nonbreedingvisitor; "?" precedingname = extinct. TINAMIFORMES TINAMIDAE Tinamus major Great Tinamou. Nothocercusbonapartei Highland Tinamou. Crypturellus soui Little Tinamou. Crypturelluscinnamomeus Thicket Tinamou. Crypturellusboucardi Slaty-breastedTinamou. Crypturellus kerriae Choco Tinamou. GAVIIFORMES GAVIIDAE Gavia stellata Red-throated Loon. Gavia arctica Arctic Loon. Gavia pacifica Pacific Loon. Gavia immer Common Loon. Gavia adamsii Yellow-billed Loon. PODICIPEDIFORMES PODICIPEDIDAE Tachybaptusdominicus Least Grebe. Podilymbuspodiceps Pied-billed Grebe. ?Podilymbusgigas Atitlan Grebe. Podicepsauritus Horned Grebe. Podicepsgrisegena Red-neckedGrebe. Podicepsnigricollis Eared Grebe. Aechmophorusoccidentalis Western Grebe. Aechmophorusclarkii Clark's Grebe. PROCELLARIIFORMES DIOMEDEIDAE Thalassarchechlororhynchos Yellow-nosed Albatross. (A) Thalassarchecauta Shy Albatross.(A) Thalassarchemelanophris Black-browed Albatross. (A) Phoebetriapalpebrata Light-mantled Albatross. (A) Diomedea exulans WanderingAlbatross. (A) Phoebastriaimmutabilis Laysan Albatross. Phoebastrianigripes Black-lootedAlbatross. Phoebastriaalbatrus Short-tailedAlbatross. (N) PROCELLARIIDAE Fulmarus glacialis Northern Fulmar. Pterodroma neglecta KermadecPetrel. (A) Pterodroma -
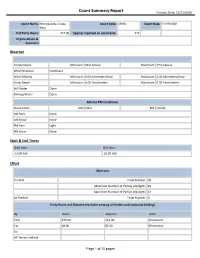
Count Summary Report Printout Date: 11/11/2016
Count Summary Report Printout Date: 11/11/2016 Count Name: Monteverde, Costa Count Code: CRMO Count Date: 12/19/2001 Rica # of Party Hours: 257.00 Species reported on count date: 376 Organizations & Sponsors: Weather Temperature Minimum: 59.0 Celsius Maximum: 77.0 Celsius Wind Direction Northeast Wind Velocity Minimum: 0.00 Kilometers/hour Maximum: 6.00 Kilometers/hour Snow Depth Minimum: 0.00 Centimeters Maximum: 0.00 Centimeters Still Water Open Moving Water Open AM and PM Conditions Cloud Cover AM: Clear PM: Cloudy AM Rain None AM Snow None PM Rain Light PM Snow None Start & End Times Start time End time 12:00 AM 10:00 AM Effort Observers In Field Total Number: 70 Minimum Number of Parties (daylight): 19 Maximum Number of Parties (daylight): 24 At Feeders Total Number: 0 Party Hours and Distance (excludes viewing at feeders and nocturnal birding) By Hours Distance Units Foot 239.00 114.00 Kilometers Car 18.00 85.00 Kilometers Air All-Terrain Vehicle Page 1 of 12 pages Count Summary Report Printout Date: 11/11/2016 Bicycle Dog Sled Golfcart Horseback Motorized Boat Non-Motorized Boat Skis/Xc-Skis Snowmachine Snowshoe Wheelchair Other Time and Distance Hours Distance Units At Feeders 0.00 Nocturnal Birding 17.00 26.00 Miles Total Party 257.00 199.00 Kilometers Checklist Species Number Number/Party Hrs. Flags Editorial Codes Highland Tinamou 3 0.0117 Great Tinamou 2 0.0078 Gray-headed Chachalaca 33 0.1284 Crested Guan 20 0.0778 Black Guan 18 0.0700 Black-breasted Wood-Quail 56 0.2179 Least Grebe 5 0.0195 Anhinga 1 0.0039 Fasciated Tiger-Heron -

Ornithological Surveys in Serranía De Los Churumbelos, Southern Colombia
Ornithological surveys in Serranía de los Churumbelos, southern Colombia Paul G. W . Salaman, Thomas M. Donegan and Andrés M. Cuervo Cotinga 12 (1999): 29– 39 En el marco de dos expediciones biológicos y Anglo-Colombian conservation expeditions — ‘Co conservacionistas anglo-colombianas multi-taxa, s lombia ‘98’ and the ‘Colombian EBA Project’. Seven llevaron a cabo relevamientos de aves en lo Serranía study sites were investigated using non-systematic de los Churumbelos, Cauca, en julio-agosto 1988, y observations and standardised mist-netting tech julio 1999. Se estudiaron siete sitios enter en 350 y niques by the three authors, with Dan Davison and 2500 m, con 421 especes registrados. Presentamos Liliana Dávalos in 1998. Each study site was situ un resumen de los especes raros para cada sitio, ated along an altitudinal transect at c. 300- incluyendo los nuevos registros de distribución más m elevational steps, from 350–2500 m on the Ama significativos. Los resultados estabilicen firme lo zonian slope of the Serranía. Our principal aim was prioridad conservacionista de lo Serranía de los to allow comparisons to be made between sites and Churumbelos, y aluco nos encontramos trabajando with other biological groups (mammals, herptiles, junto a los autoridades ambientales locales con insects and plants), and, incorporating geographi cuiras a lo protección del marcizo. cal and anthropological information, to produce a conservation assessment of the region (full results M e th o d s in Salaman et al.4). A sizeable part of eastern During 14 July–17 August 1998 and 3–22 July 1999, Cauca — the Bota Caucana — including the 80-km- ornithological surveys were undertaken in Serranía long Serranía de los Churumbelos had never been de los Churumbelos, Department of Cauca, by two subject to faunal surveys. -

Ultimate Bolivia Tour Report 2019
Titicaca Flightless Grebe. Swimming in what exactly? Not the reed-fringed azure lake, that’s for sure (Eustace Barnes) BOLIVIA 8 – 29 SEPTEMBER / 4 OCTOBER 2019 LEADER: EUSTACE BARNES Bolivia, indeed, THE land of parrots as no other, but Cotingas as well and an astonishing variety of those much-loved subfusc and generally elusive denizens of complex uneven surfaces. Over 700 on this tour now! 1 BirdQuest Tour Report: Ultimate Bolivia 2019 www.birdquest-tours.com Blue-throated Macaws hoping we would clear off and leave them alone (Eustace Barnes) Hopefully, now we hear of colourful endemic macaws, raucous prolific birdlife and innumerable elusive endemic denizens of verdant bromeliad festooned cloud-forests, vast expanses of rainforest, endless marshlands and Chaco woodlands, each ringing to the chorus of a diverse endemic avifauna instead of bleak, freezing landscapes occupied by impoverished unhappy peasants. 2 BirdQuest Tour Report: Ultimate Bolivia 2019 www.birdquest-tours.com That is the flowery prose, but Bolivia IS that great destination. The tour is no longer a series of endless dusty journeys punctuated with miserable truck-stop hotels where you are presented with greasy deep-fried chicken and a sticky pile of glutinous rice every day. The roads are generally good, the hotels are either good or at least characterful (in a good way) and the food rather better than you might find in the UK. The latter perhaps not saying very much. Palkachupe Cotinga in the early morning light brooding young near Apolo (Eustace Barnes). That said, Bolivia has work to do too, as its association with that hapless loser, Che Guevara, corruption, dust and drug smuggling still leaves the country struggling to sell itself. -

Lepidocolaptes Angustirostris) in a Southern Temperate Forest of Central-East Argentina
Studies on Neotropical Fauna and Environment ISSN: 0165-0521 (Print) 1744-5140 (Online) Journal homepage: https://www.tandfonline.com/loi/nnfe20 Nesting biology of the Narrow-billed Woodcreeper (Lepidocolaptes angustirostris) in a southern temperate forest of central-east Argentina Adrián Jauregui, Exequiel Gonzalez & Luciano N. Segura To cite this article: Adrián Jauregui, Exequiel Gonzalez & Luciano N. Segura (2019): Nesting biology of the Narrow-billed Woodcreeper (Lepidocolaptesangustirostris) in a southern temperate forest of central-east Argentina, Studies on Neotropical Fauna and Environment To link to this article: https://doi.org/10.1080/01650521.2019.1590968 Published online: 25 Mar 2019. Submit your article to this journal View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=nnfe20 STUDIES ON NEOTROPICAL FAUNA AND ENVIRONMENT https://doi.org/10.1080/01650521.2019.1590968 ORIGINAL ARTICLE Nesting biology of the Narrow-billed Woodcreeper (Lepidocolaptes angustirostris) in a southern temperate forest of central-east Argentina Adrián Jauregui, Exequiel Gonzalez and Luciano N. Segura Sección Ornitología, Museo de La Plata, Universidad Nacional de La Plata-CONICET, La Plata, Buenos Aires, Argentina ABSTRACT ARTICLE HISTORY We present data on the nesting biology of the Narrow-billed Woodcreeper (Lepidocolaptes Received 24 September 2018 angustirostris) in a natural forest in central-east Argentina. A total of 18 nests were found during Accepted 27 February 2019 – four breeding seasons (2015 2019; from September to January), located in cavities (natural, KEYWORDS artificial and woodpecker cavities). The incubation period lasted 16 days and eggs were larger Breeding parameters; than those from northern populations. -

Predation on Vertebrates by Neotropical Passerine Birds Leonardo E
Lundiana 6(1):57-66, 2005 © 2005 Instituto de Ciências Biológicas - UFMG ISSN 1676-6180 Predation on vertebrates by Neotropical passerine birds Leonardo E. Lopes1,2, Alexandre M. Fernandes1,3 & Miguel Â. Marini1,4 1 Depto. de Biologia Geral, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, 31270-910, Belo Horizonte, MG, Brazil. 2 Current address: Lab. de Ornitologia, Depto. de Zoologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Av. Antônio Carlos, 6627, Pampulha, 31270-910, Belo Horizonte, MG, Brazil. E-mail: [email protected]. 3 Current address: Coleções Zoológicas, Aves, Instituto Nacional de Pesquisas da Amazônia, Avenida André Araújo, 2936, INPA II, 69083-000, Manaus, AM, Brazil. E-mail: [email protected]. 4 Current address: Lab. de Ornitologia, Depto. de Zoologia, Instituto de Biologia, Universidade de Brasília, 70910-900, Brasília, DF, Brazil. E-mail: [email protected] Abstract We investigated if passerine birds act as important predators of small vertebrates within the Neotropics. We surveyed published studies on bird diets, and information on labels of museum specimens, compiling data on the contents of 5,221 stomachs. Eighteen samples (0.3%) presented evidence of predation on vertebrates. Our bibliographic survey also provided records of 203 passerine species preying upon vertebrates, mainly frogs and lizards. Our data suggest that vertebrate predation by passerines is relatively uncommon in the Neotropics and not characteristic of any family. On the other hand, although rare, the ability to prey on vertebrates seems to be widely distributed among Neotropical passerines, which may respond opportunistically to the stimulus of a potential food item. -

Birds of Brazil
BIRDS OF BRAZIL - MP3 SOUND COLLECTION version 2.0 List of recordings 0001 1 Greater Rhea 1 Song 0:17 Rhea americana (20/7/2005, Chapada dos Guimaraes, Mato Grosso, Brazil, 15.20S,55.50W) © Peter Boesman 0006 1 Gray Tinamou 1 Song 0:43 Tinamus tao (15/8/2007 18:30h, Nirgua area, San Felipe, Venezuela, 10.15N,68.30W) © Peter Boesman 0006 2 Gray Tinamou 2 Song 0:24 Tinamus tao (2/1/2008 17:15h, Tarapoto tunnel road, San Martín, Peru, 06.25S,76.15W) © Peter Boesman 0006 3 Gray Tinamou 3 Whistle 0:09 Tinamus tao (15/8/2007 18:30h, Nirgua area, San Felipe, Venezuela, 10.15N,68.30W) © Peter Boesman 0007 1 Solitary Tinamou 1 Song () 0:05 Tinamus solitarius (11/8/2004 08:00h, Serra da Graciosa, Paraná, Brazil, 25.20S,48.55W) © Peter Boesman. 0009 1 Great Tinamou 1 Song 1:31 Tinamus major (3/1/2008 18:45h, Morro de Calzada, San Martín, Peru, 06.00S,77.05W) © Peter Boesman 0009 2 Great Tinamou 2 Song 0:31 Tinamus major (28/7/2009 18:00h, Pantiacolla Lodge, Madre de Dios, Peru, 12.39S,71.14W) © Peter Boesman 0009 3 Great Tinamou 3 Song 0:27 Tinamus major (26/7/2009 17:00h, Pantiacolla Lodge, Madre de Dios, Peru, 12.39S,71.14W) © Peter Boesman 0009 4 Great Tinamou 4 Song 0:46 Tinamus major (22nd July 2010 17h00, ACTS Explornapo, Loreto, Peru, 120 m. 3°10' S, 72°55' W). (Background: Thrush-like Antpitta, Elegant Woodcreeper). © Peter Boesman. 0009 5 Great Tinamou 5 Call 0:11 Tinamus major (17/7/2006 17:30h, Iracema falls, Presidente Figueiredo, Amazonas, Brazil, 02.00S,60.00W) © Peter Boesman.