Taking "Pandemic" Seriously: Making the Black Death Global
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plague Manual for Investigation
Plague Summary Plague is a flea-transmitted bacterial infection of rodents caused by Yersinia pestis. Fleas incidentally transmit the infection to humans and other susceptible mammalian hosts. Humans may also contract the disease from direct contact with an infected animal. The most common clinical form is acute regional lymphadenitis, called bubonic plague. Less common clinical forms include septicemic, pneumonic, and meningeal plague. Pneumonic plague can be spread from person to person via airborne transmission, potentially leading to epidemics of primary pneumonic plague. Plague is immediately reportable to the New Mexico Department of Health. Plague is treatable with antibiotics, but has a high fatality rate with inadequate or delayed treatment. Plague preventive measures include: isolation of pneumonic plague patients; prophylactic treatment of pneumonic case contacts; avoiding contact with rodents and their fleas; reducing rodent harborage around the home; using flea control on pets; and, preventing pets from hunting. Agent Plague is caused by Yersinia pestis, a gram-negative, bi-polar staining, non-motile, non-spore forming coccobacillus. Transmission Reservoir: Wild rodents (especially ground squirrels) are the natural vertebrate reservoir of plague. Lagomorphs (rabbits and hares), wild carnivores, and domestic cats may also be a source of infection to humans. Vector: In New Mexico, the rock squirrel flea, Oropsylla montana, is the most important vector of plague for humans. Many more flea species are involved in the transmission of sylvatic (wildlife) plague. Mode of Transmission: Most humans acquire plague through the bites of infected fleas. Fleas can be carried into the home by pet dogs and cats, and may be abundant in woodpiles or burrows where peridomestic rodents such as rock squirrels (Spermophilus variegatus) have succumbed to plague infection. -

CASE REPORT the PATIENT 33-Year-Old Woman
CASE REPORT THE PATIENT 33-year-old woman SIGNS & SYMPTOMS – 6-day history of fever Katherine Lazet, DO; – Groin pain and swelling Stephanie Rutterbush, MD – Recent hiking trip in St. Vincent Ascension Colorado Health, Evansville, Ind (Dr. Lazet); Munson Healthcare Ostego Memorial Hospital, Lewiston, Mich (Dr. Rutterbush) [email protected] The authors reported no potential conflict of interest THE CASE relevant to this article. A 33-year-old Caucasian woman presented to the emergency department with a 6-day his- tory of fever (103°-104°F) and right groin pain and swelling. Associated symptoms included headache, diarrhea, malaise, weakness, nausea, cough, and anorexia. Upon presentation, she admitted to a recent hike on a bubonic plague–endemic trail in Colorado. Her vital signs were unremarkable, and the physical examination demonstrated normal findings except for tender, erythematous, nonfluctuant right inguinal lymphadenopathy. The patient was admitted for intractable pain and fever and started on intravenous cefoxitin 2 g IV every 8 hours and oral doxycycline 100 mg every 12 hours for pelvic inflammatory disease vs tick- or flea-borne illness. Due to the patient’s recent trip to a plague-infested area, our suspicion for Yersinia pestis infection was high. The patient’s work-up included a nega- tive pregnancy test and urinalysis. A com- FIGURE 1 plete blood count demonstrated a white CT scan from admission blood cell count of 8.6 (4.3-10.5) × 103/UL was revealing with a 3+ left shift and a platelet count of 112 (180-500) × 103/UL. A complete metabolic panel showed hypokalemia and hyponatremia (potassium 2.8 [3.5-5.1] mmol/L and sodium 134 [137-145] mmol/L). -

Plague Surveillance Protocol
December 2006 Plague Surveillance Protocol Plague may occur from an unintentional exposure to infected rodents and their fleas or through an intentional exposure such as a bioterrorism (BT) event. When necessary this protocol addresses unintentional and intentional exposures separately; otherwise the protocol applies to both situations. This protocol applies if a case of plague is highly suspected and does not apply to non-specific pulmonary, gastrointestinal, or rash illnesses. Provider Responsibilities 1) Report suspect or confirmed cases of plague immediately by phone to the local health department where the patient is a resident. Complete the provider (yellow) section of the WVEDSS form and forward to the local health department. 2) Notify the infection control practitioner immediately for hospitalized patients. Assure that the case-patient is appropriately isolated using standard and droplet precautions. 3) Assure that the laboratory forwards any isolates of Yersinia pestis to the Office of Laboratory Services for confirmation. The Office of Laboratory Services is available at (304)-558-3530 or on the web at: http://www.wvdhhr.org/labservices/index.cfm 4) Plan to collaborate with public health officials to identify close contacts of patients with pneumonic plague (unprotected face-to-face exposure within 3 feet of the case-patient), including health care workers. Laboratory Responsibilities 1) Report suspect or confirmed Yersinia pestis immediately to: a) The physician; b) The infection control practitioner; and c) The local health department. 2) Reports to the health department should include: Name, full address, date of birth, specimen source, test performed, test result and normal value. Fax a laboratory report or a completed ‘yellow card’ to the local health department. -

Adaptive Response of Yersinia Pestis to Extracellular Effectors of Innate Immunity During Bubonic Plague
Adaptive response of Yersinia pestis to extracellular effectors of innate immunity during bubonic plague Florent Sebbane*†, Nadine Lemaıˆtre*‡§, Daniel E. Sturdevant¶, Roberto Rebeil*ʈ, Kimmo Virtaneva¶, Stephen F. Porcella¶, and B. Joseph Hinnebusch*,** *Laboratory of Zoonotic Pathogens and ¶Genomics Core Facility, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, MT 59840; ‡Institut National de la Sante´et de la Recherche Me´dicale Unite´801 and Faculte´deMe´ decine Henri Warembourg, Universite´de Lille II, Lille F-59045, France; and §Institut Pasteur, Lille F-59021, France Edited by John J. Mekalanos, Harvard Medical School, Boston, MA, and approved June 13, 2006 (received for review February 11, 2006) Yersinia pestis causes bubonic plague, characterized by an en- phagocytic oxidative burst that generates antimicrobial reactive larged, painful lymph node, termed a bubo, that develops after oxygen species (ROS), down-regulate the normal proinflamma- bacterial dissemination from a fleabite site. In susceptible animals, tory response, and induce apoptosis of dendritic cells, NK cells, the bacteria rapidly escape containment in the lymph node, spread macrophages, and polymorphonuclear neutrophils (PMNs) (3, 4, systemically through the blood, and produce fatal sepsis. The 6–8). Although substantial, most of the experimental evidence fulminant progression of disease has been largely ascribed to the for these models comes from in vitro studies with the less virulent ability of Y. pestis to avoid phagocytosis and exposure to antimi- enteric pathogens Yersinia pseudotuberculosis and Yersinia en- crobial effectors of innate immunity. In vivo microarray analysis of terocolitica or from studies using attenuated strains of Y. -

History of Infectious Diseases
1 Chapter 1 History of Infectious Diseases Maria Ines Zanoli Sato CETESB, São Paulo, Brazil ABSTRACT This chapter provides a review of infectious disease to date and the challenges they may present in the future. The main pandemics that have driven the history of humanity are described, from the first to be recorded in 3180 BC to more recent ones such as AIDIS, SARS and others associated with emerging pathogens. The essential role of emerging scientific specialisms (particularly microbiology, public health and sanitary engineering) to our understanding of the causes of these diseases (and how they may be better monitored, controlled and prevented) is presented. Globalization and climate change, determin- ing factors for the ecology of infectious diseases and their emergence and re-emergence, are discussed and point to the urgent need for research to deal with these threats that continue to have a significant impact on human development and wellbeing. INTRODUCTION In recent decades, concern about the quality and sustainability of the environment has been increasing and this is now a critical global issue, involving both industrialised and low-income countries. The influence of the environment on human health dates back to antiquity, when human life expectancy was shorter than today as a result of the hostile environment in which they lived. Infectious diseases have been a health hazard faced by humanity since human beings first appeared. These diseases spread from person to person or from animals to humans, often through water, food or insect vectors and so transmis- sion may be directly affected by environmental changes (Yassi et al., 2001). -

WHO/CDS/CSR/EDC/99.2 Plague Manual: Epidemiology
WHO/CDS/CSR/EDC/99.2 Plague Manual: Epidemiology, Distribution, Surveillance and Control World Health Organization Communicable Disease Surveillance and Response This document has been downloaded from the WHO/EMC Web site. The original cover pages and lists of participants are not included. See http://www.who.int/emc for more information. © World Health Organization This document is not a formal publication of the World Health Organization (WHO), and all rights are reserved by the Organization. The document may, however, be freely reviewed, abstracted, reproduced and translated, in part or in whole, but not for sale nor for use in conjunction with commercial purposes. The views expressed in documents by named authors are solely the responsibility of those authors. The mention of specific companies or specific manufacturers' products does no imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. WHO/CDS/CSR/EDC/99.2 Distribution : General Original : English Plague Manual Epidemiology, Distribution, Surveillance and Control PRINCIPAL AUTHORS Dr David T. Dennis and Dr Kenneth L. Gage National Center for Infectious Diseases Centers for Disease Control and Prevention Fort Collins, Colorado, USA Dr Norman Gratz, World Health Organization, Geneva, Switzerland Dr Jack D. Poland, Colorado State University, Colorado, USA Dr Evgueni Tikhomirov, World Health Organization, Geneva, Switzerland WHO/CDS/CSR/EDC/99.2 Plague Manual Epidemiology, Distribution, Surveillance -

Plague Disease Fact Sheet Series
WISCONSIN DIVISION OF PUBLIC HEALTH Department of Health Services Plague Disease Fact Sheet Series What is plague? Plague is an infectious disease that affects animals and humans. It is caused by the bacterium Yersinia pestis. This bacterium is found in rodents and their fleas and occurs in many areas of the world, including the United States. How do people get plague? Plague is usually acquired from the bites of fleas infected with the plague bacterium. Fleas become infected by feeding on rodents, such as chipmunks, prairie dogs, ground squirrels, mice, and other mammals that are infected with the Yersinia pestis bacterium. These fleas can then transmit the bacteria when they subsequently bite humans. Less commonly, plague can be acquired from being bitten or scratched by infected animals or by handling carcasses of animals (often rodents or rabbits) that had been infected. Persons can also become infected by inhaling respiratory droplets from a coughing person who has plague pneumonia. What are the signs and symptoms of plague? The typical sign of the most common form of human plague is a swollen and very tender lymph node, accompanied by pain. The swollen node is called a "bubo" (hence the term "bubonic plague"). Besides the swollen lymph node, bubonic plague symptoms include fever, chills, headache, and extreme exhaustion. A person usually becomes ill with bubonic plague 2-6 days after being infected. When bubonic plague is left untreated, plague bacteria invade the bloodstream. When plague bacteria multiply in the bloodstream, they spread rapidly throughout the body and cause a severe and often fatal condition. -
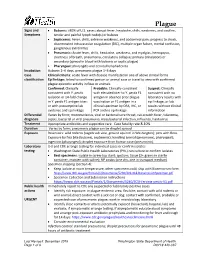
Plague Reporting and Investigation Guideline
Plague Signs and • Bubonic: >80% of U.S. cases; abrupt fever, headache, chills, weakness, and swollen, Symptoms tender and painful lymph node(s) or buboes • Septicemic: Fever, chills, extreme weakness, and abdominal pain; progress to shock, disseminated intravascular coagulation (DIC), multiple organ failure, mental confusion, gangrenous extremities • Pneumonic: Acute fever, chills, headache, weakness, and myalgias, hemoptysis, shortness of breath, pneumonia, circulatory collapse; primary (inhalation) or secondary (spread in blood with bubonic or septic plague) • Pharyngeal: pharyngitis and cervical lymphadenitis Incubation Bubonic 2–6 days, pneumonic plague 1–3 days Case Clinical criteria: acute fever with disease manifested in one of above clinical forms classification Epi linkage: linked to confirmed person or animal case or travel to area with confirmed plague epizootic activity in fleas or animals Confirmed: Clinically Probable: Clinically consistent Suspect: Clinically consistent with Y. pestis with elevated titer to Y. pestis F1 consistent with no isolation or ≥4-fold change antigen in absence prior plague laboratory results with in Y. pestis F1 antigen titer; vaccination or F1 antigen in a epi linkage, or lab or with presumptive lab clinical specimen by DFA, IHC, or results without clinical evidence and epi linkage PCR and no epi linkage information Differential Varies by form; mononucleosis, viral or bacterial sore throat, cat-scratch fever, tularemia, diagnosis sepsis, bacterial or viral pneumonia, mycobacterial infection, influenza, hantavirus Treatment Appropriate antibiotics and supportive care. Case fatality rate 8-10%. Duration Varies by form; pneumonic plague can be droplet spread Exposure Reservoirs: wild rodents (sagebrush vole, ground squirrel in Washington); pets with fleas. Exposure by flea bite (bubonic, septicemic); handling animal (pneumonic, pharyngeal); ingestion (pharyngeal); droplet exposure from human case (pneumonic). -

The Bubonic Plague Outbreak of 1900 Charles Mcclain
Of Medicine, Race, and American Law: The Bubonic Plague Outbreak of 1900 Charles McClain In March of 1900, several cases of bubonic plague were discovered in San Francisco'sChinatown. In response the health authorities,at the instance of the Surgeon General of the United State; sought to implement a series of extraordinarilycoercive measures aimed at the city's Asian inhabitants. The measures provoked an uproar among the Chinese and they determined to challenge them in the federal Circuit Courtfor the Northern District of Cali- fornia. This essay, based on extensive research in court records, the archives of the U.S. Public Health Service, and press accounts in English and Chi- nese, documents the complex events that gave rise to the cases of Wong Wai v. Williamson and Jew Ho v. Williamson and the cases themselves as they unfolded in the courts. The cases raised new and difficult questions of fact and of law and tested as few other cases have before or since a court's capac- ity to act as arbiterbetween individual rights (and the rights of an ostracized minority at that) and the public interest in a period of acute health emergency. In the spring of 1900 several cases of bubonic plague were detected in the Chinese quarter of San Francisco. In response, the federal and local health authorities sought to implement a series of measures unparalleled in Charles McClain is vice chairman of the Jurisprudence, and Social Policy Program and lecturer, School of Law (Boalt Hall), University of California, Berkeley. Ph.D. 1972, Stanford University;, J.D. -

1 Plague 1. the First Pandemic, Known As the Justinian Plague
Plague 1. The first pandemic, known as the Justinian plague (AD 541-544), began in Egypt and spread throughout the Middle East and Mediterranean areas. Eventually, the entire known world was affected. By the 8th century, plague receded into scattered endemic areas. 2. The second pandemic began in 1347, when traders from central Asia introduced plague into ports of Sicily. This became the first epidemic, known as the Black Death, which killed over one third of the population of Europe. Later, following the Great Plague of London (1665), the disease subsided. 3. The third pandemic began in Hong Kong in 1894 and continues to the present. Y pestis: nonmotile, non–spore-forming, pleomorphic, gram-negative coccobacillus. The bacteria elaborate a lipopolysaccharide endotoxin, coagulase, and a fibrinolysin, which are the principal factors in the pathogenesis of this disease. Domestic and urban rats are the most important reservoirs for the plague bacillus, but field mice, cats, camels, chipmunks, prairie dogs, rabbits, and squirrels can be important animal reservoirs as well. The most important vector for transmission of plague is the rat flea, Xenopsylla cheopis. Ticks and human lice have been identified as possible vectors. Humans are accidental hosts in the natural cycle of this disease. The classic mode of transmission to humans is a fleabite. Alternately, broken skin serves as a portal when tissue or blood of an infected animal is handled (skinning or evisceration of infected animals). Pneumonic plague occurs when pneumonia results from either hematogenous spread (secondary pneumonic plague) or inhalation (primary pneumonic plague) of organisms transmitted from animals or other humans. -

The Year of the Rat
THE YEAR OF THE RAT: A SOCIAL AND MEDICAL HISTORY OF BUBONIC PLAGUE IN SAN FRANCISCO, 1900-1908 __________________ University Thesis Presented to the Faculty of California State University, East Bay __________________ In Partial Fulfillment of the Requirements for the Degree Master of Arts in History __________________ By Jennifer Nicole Faggiano May, 2019 Copyright © 2019 by Jenifer Nicole Faggiano ii TABLE OF CONTENTS Copyright ............................................................................................................................ii List of Figures and Photos ..................................................................................................v Introduction .........................................................................................................................1 Chapter 1. Gold Mountain: The Chinese In California During the 18th century............................................................................................................. 12 Chapter 2. The Menagerie: The Chinese in San Francisco ............................................... 37 Chapter 3. La Piqure de Puce: Plague History and Ecology............................................. 51 Chapter 4. Fallen Leaves: The Arrival of Plague In San Francisco.................................. 67 Chapter 5. Fake News: Death, Denial, and Kinyoun’s Attempt to End the Outbreak ........................................................................................................... 83 Chapter 6. Ashes to Ashes: Rupert Blue, the End -

Zoonoses and Communicable Diseases Common to Man and Animals
ZOONOSES AND COMMUNICABLE DISEASES COMMON TO MAN AND ANIMALS Third Edition Volume I Bacterioses and Mycoses Scientific and Technical Publication No. 580 PAN AMERICAN HEALTH ORGANIZATION Pan American Sanitary Bureau, Regional Office of the WORLD HEALTH ORGANIZATION 525 Twenty-third Street, N.W. Washington, D.C. 20037 U.S.A. 2001 Also published in Spanish (2001) with the title: Zoonosis y enfermedades transmisibles comunes al hombre a los animales ISBN 92 75 31580 9 PAHO Cataloguing-in-Publication Pan American Health Organization Zoonoses and communicable diseases common to man and animals 3rd ed. Washington, D.C.: PAHO, © 2001. 3 vol.—(Scientific and Technical Publication No. 580) ISBN 92 75 11580 X I. Title II. Series 1. ZOONOSES 2. BACTERIAL INFECTIONS AND MYCOSES 3. COMMUNICABLE DISEASE CONTROL 4. FOOD CONTAMINATION 5. PUBLIC HEALTH VETERINARY 6. DISEASE RESERVOIRS NLM WC950.P187 2001 En The Pan American Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and inquiries should be addressed to the Publications Program, Pan American Health Organization, Washington, D.C., U.S.A., which will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available. ©Pan American Health Organization, 2001 Publications of the Pan American Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. All rights are reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the Pan American Health Organization concerning the status of any country, ter- ritory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.