Effects of Exsanguination on the Optic Nerve Head
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors
pharmaceutics Article Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors Hyeong Min Kim 1,†, Hyounkoo Han 2,†, Hye Kyoung Hong 1, Ji Hyun Park 1, Kyu Hyung Park 1, Hyuncheol Kim 2,* and Se Joon Woo 1,* 1 Department of Ophthalmology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam 13620, Korea; [email protected] (H.M.K.); [email protected] (H.K.H.); [email protected] (J.H.P.); [email protected] (K.H.P.) 2 Department of Chemical and Biomolecular Engineering, Sogang University, Seoul 04107, Korea; [email protected] * Correspondence: [email protected] (H.K.); [email protected] (S.J.W.); Tel.: +82-2-705-8922 (H.K.); +82-31-787-7377 (S.J.W.); Fax: +82-2-3273-0331 (H.K.); +82-31-787-4057 (S.J.W.) † These authors contributed equally to this work. Abstract: In this study, Retina-RPE-Choroid-Sclera (RCS) and RPE-Choroid-Sclera (CS) were prepared by scraping them off neural retina, and using the Ussing chamber we measured the average time– concentration values in the acceptor chamber across five isolated rabbit tissues for each drug molecule. We determined the outward direction permeability of the RCS and CS and calculated the neural retina permeability. The permeability coefficients of RCS and CS were as follows: ganciclovir, 13.78 ± 5.82 and 23.22 ± 9.74; brimonidine, 15.34 ± 7.64 and 31.56 ± 12.46; bevacizumab, 0.0136 ± 0.0059 and 0.0612 ± 0.0264 (×10−6 cm/s). -

Localization of S-100 Protein in Mulier Cells of the Retina— 1
Reports Localization of S-100 Protein in Mulier Cells of the Retina— 1. Light Microscopical Immunocytochemistry G. Terenghi,* D. Cocchia,f F. Micherti,f A. R. T. Pererson4 D. F. Cole,§ S. R. Bloom,11 ond J. M. Polok* S-100 is an acidic brain protein previously found to be pres- constituent and could be involved in the functions ent in glial cells of the brain and the nervous system of gut of normal and diseased retina10"; their identification and respiratory tract. Immunocytochemistry at the light by the use of a suitable marker is thus of primary microscopical level localized immunoreactivity for S-100 in relevance. In this study, we report on the immuno- the Mulier cells in the retina of rat, guinea pig, and Chinese cytochemical visualization at light microscopic level hamster. The Mulier cells represent the main glial com- ponent of the retina, with a structural role in the support of Mulier cells in the mammalian retina by using the and insulation of neurons and sensory elements. The use presence of S-100 protein as a marker for glial cells. of S-100 protein as an immunocytochemical marker of Materials and Methods. Albino rats (n = 5), guinea Miiller cells may be useful in the study of pathologic con- pigs (n = 5), and Chinese hamsters (n = 4) were used. ditions of the retina where glial cell proliferation could re- The animals were killed by exsanguination under flect the index of neuronal injury. Invest Ophthalmol Vis ether anaesthesia; the eyeballs were removed and im- Sci 24:976-980, 1983 mediately processed. -

The Complexity and Origins of the Human Eye: a Brief Study on the Anatomy, Physiology, and Origin of the Eye
Running Head: THE COMPLEX HUMAN EYE 1 The Complexity and Origins of the Human Eye: A Brief Study on the Anatomy, Physiology, and Origin of the Eye Evan Sebastian A Senior Thesis submitted in partial fulfillment of the requirements for graduation in the Honors Program Liberty University Spring 2010 THE COMPLEX HUMAN EYE 2 Acceptance of Senior Honors Thesis This Senior Honors Thesis is accepted in partial fulfillment of the requirements for graduation from the Honors Program of Liberty University. ______________________________ David A. Titcomb, PT, DPT Thesis Chair ______________________________ David DeWitt, Ph.D. Committee Member ______________________________ Garth McGibbon, M.S. Committee Member ______________________________ Marilyn Gadomski, Ph.D. Assistant Honors Director ______________________________ Date THE COMPLEX HUMAN EYE 3 Abstract The human eye has been the cause of much controversy in regards to its complexity and how the human eye came to be. Through following and discussing the anatomical and physiological functions of the eye, a better understanding of the argument of origins can be seen. The anatomy of the human eye and its many functions are clearly seen, through its complexity. When observing the intricacy of vision and all of the different aspects and connections, it does seem that the human eye is a miracle, no matter its origins. Major biological functions and processes occurring in the retina show the intensity of the eye’s intricacy. After viewing the eye and reviewing its anatomical and physiological domain, arguments regarding its origins are more clearly seen and understood. Evolutionary theory, in terms of Darwin’s thoughts, theorized fossilization of animals, computer simulations of eye evolution, and new research on supposed prior genes occurring in lower life forms leading to human life. -

Foveola Nonpeeling Internal Limiting Membrane Surgery to Prevent Inner Retinal Damages in Early Stage 2 Idiopathic Macula Hole
Graefes Arch Clin Exp Ophthalmol DOI 10.1007/s00417-014-2613-7 RETINAL DISORDERS Foveola nonpeeling internal limiting membrane surgery to prevent inner retinal damages in early stage 2 idiopathic macula hole Tzyy-Chang Ho & Chung-May Yang & Jen-Shang Huang & Chang-Hao Yang & Muh-Shy Chen Received: 29 October 2013 /Revised: 26 February 2014 /Accepted: 5 March 2014 # Springer-Verlag Berlin Heidelberg 2014 Abstract Keywords Fovea . Foveola . Internal limiting membrane . Purpose The purpose of this study was to investigate and macular hole . Müller cell . Vitrectomy present the results of a new vitrectomy technique to preserve the foveolar internal limiting membrane (ILM) during ILM peeling in early stage 2 macular holes (MH). Introduction Methods The medical records of 28 consecutive patients (28 eyes) with early stage 2 MH were retrospectively reviewed It is generally agreed that internal limiting membrane (ILM) and randomly divided into two groups by the extent of ILM peeling is important in achieving closure of macular holes peeing. Group 1: foveolar ILM nonpeeling group (14 eyes), (MH) [1]. An autopsy study of a patient who had undergone and group 2: total peeling of foveal ILM group (14 eyes). A successful MH closure showed an area of absent ILM sur- donut-shaped ILM was peeled off, leaving a 400-μm-diameter rounding the sealed MH [2]. ILM over foveola in group 1. The present ILM peeling surgery of idiopathic MH in- Results Smooth and symmetric umbo foveolar contour was cludes total removal of foveolar ILM. However, removal of restored without inner retinal dimpling in all eyes in group 1, all the ILM over the foveola causes anatomical changes of the but not in group 2. -

Embryology, Anatomy, and Physiology of the Afferent Visual Pathway
CHAPTER 1 Embryology, Anatomy, and Physiology of the Afferent Visual Pathway Joseph F. Rizzo III RETINA Physiology Embryology of the Eye and Retina Blood Supply Basic Anatomy and Physiology POSTGENICULATE VISUAL SENSORY PATHWAYS Overview of Retinal Outflow: Parallel Pathways Embryology OPTIC NERVE Anatomy of the Optic Radiations Embryology Blood Supply General Anatomy CORTICAL VISUAL AREAS Optic Nerve Blood Supply Cortical Area V1 Optic Nerve Sheaths Cortical Area V2 Optic Nerve Axons Cortical Areas V3 and V3A OPTIC CHIASM Dorsal and Ventral Visual Streams Embryology Cortical Area V5 Gross Anatomy of the Chiasm and Perichiasmal Region Cortical Area V4 Organization of Nerve Fibers within the Optic Chiasm Area TE Blood Supply Cortical Area V6 OPTIC TRACT OTHER CEREBRAL AREASCONTRIBUTING TO VISUAL LATERAL GENICULATE NUCLEUSPERCEPTION Anatomic and Functional Organization The brain devotes more cells and connections to vision lular, magnocellular, and koniocellular pathways—each of than any other sense or motor function. This chapter presents which contributes to visual processing at the primary visual an overview of the development, anatomy, and physiology cortex. Beyond the primary visual cortex, two streams of of this extremely complex but fascinating system. Of neces- information flow develop: the dorsal stream, primarily for sity, the subject matter is greatly abridged, although special detection of where objects are and for motion perception, attention is given to principles that relate to clinical neuro- and the ventral stream, primarily for detection of what ophthalmology. objects are (including their color, depth, and form). At Light initiates a cascade of cellular responses in the retina every level of the visual system, however, information that begins as a slow, graded response of the photoreceptors among these ‘‘parallel’’ pathways is shared by intercellular, and transforms into a volley of coordinated action potentials thalamic-cortical, and intercortical connections. -

Outer Retinal Morphology and Visual Function in Patients with Idiopathic Epiretinal Membrane
CLINICAL SCIENCES Outer Retinal Morphology and Visual Function in Patients With Idiopathic Epiretinal Membrane Ken Watanabe, MD; Kazushige Tsunoda, MD, PhD; Yoshinobu Mizuno, MD; Kunihiko Akiyama, MD; Toru Noda, MD Objective: To determine the relationship between the uted to the BCVA. The standardized partial regression morphology of the fovea and visual acuity in patients with coefficient  was 0.415 for the COST line, 0.287 for the an untreated idiopathic epiretinal membrane (ERM). IS/OS junction line, and 0.247 for the external limiting membrane. However, the other features, eg, foveal bulge, Methods: We examined 52 eyes of 45 patients diag- inner limiting membrane, foveal pit, and ERM, were not nosed with an ERM. The morphology of the foveal area was significantly associated with the BCVA. The central reti- determined by spectral-domain optical coherence tomog- nal thickness was significantly correlated with the BCVA raphy. The relationships between the best-corrected vi- (r2=0.274; PϽ.01). sual acuity (BCVA) and 8 optical coherence tomography features, central retinal thickness, cone outer segment tip Conclusions: At an early stage of an ERM, only the pho- (COST) line, photoreceptor inner/outer segment (IS/OS) toreceptor structures are significantly associated with the junction line, foveal bulge of the IS/OS line, external lim- BCVA, and the appearance of the COST line was most iting membrane, inner limiting membrane, foveal pit, and highly associated. Detailed examinations of the photo- ERM over the foveal center, were evaluated. receptor structures using optical coherence tomogra- phy may help find photoreceptor dysfunction in cases Results: Multiple regression analysis showed that in- of idiopathic ERM. -

Anatomy and Physiology of the Afferent Visual System
Handbook of Clinical Neurology, Vol. 102 (3rd series) Neuro-ophthalmology C. Kennard and R.J. Leigh, Editors # 2011 Elsevier B.V. All rights reserved Chapter 1 Anatomy and physiology of the afferent visual system SASHANK PRASAD 1* AND STEVEN L. GALETTA 2 1Division of Neuro-ophthalmology, Department of Neurology, Brigham and Womens Hospital, Harvard Medical School, Boston, MA, USA 2Neuro-ophthalmology Division, Department of Neurology, Hospital of the University of Pennsylvania, Philadelphia, PA, USA INTRODUCTION light without distortion (Maurice, 1970). The tear–air interface and cornea contribute more to the focusing Visual processing poses an enormous computational of light than the lens does; unlike the lens, however, the challenge for the brain, which has evolved highly focusing power of the cornea is fixed. The ciliary mus- organized and efficient neural systems to meet these cles dynamically adjust the shape of the lens in order demands. In primates, approximately 55% of the cortex to focus light optimally from varying distances upon is specialized for visual processing (compared to 3% for the retina (accommodation). The total amount of light auditory processing and 11% for somatosensory pro- reaching the retina is controlled by regulation of the cessing) (Felleman and Van Essen, 1991). Over the past pupil aperture. Ultimately, the visual image becomes several decades there has been an explosion in scientific projected upside-down and backwards on to the retina understanding of these complex pathways and net- (Fishman, 1973). works. Detailed knowledge of the anatomy of the visual The majority of the blood supply to structures of the system, in combination with skilled examination, allows eye arrives via the ophthalmic artery, which is the first precise localization of neuropathological processes. -

Review Article Current Trends About Inner Limiting Membrane Peeling in Surgery for Epiretinal Membranes
Hindawi Publishing Corporation Journal of Ophthalmology Volume 2015, Article ID 671905, 13 pages http://dx.doi.org/10.1155/2015/671905 Review Article Current Trends about Inner Limiting Membrane Peeling in Surgery for Epiretinal Membranes Francesco Semeraro,1 Francesco Morescalchi,1 Sarah Duse,1 Elena Gambicorti,1 Andrea Russo,1 and Ciro Costagliola2,3 1 Department of Medical and Surgical Specialties, Radiological Specialties and Public Health, Ophthalmology Clinic, University of Brescia, 25123 Brescia, Italy 2Department of Medicine and Health Sciences, University of Molise, 86100 Campobasso, Italy 3I.R.C.C.S. Neuromed, LocalitaCamerelle,Pozzilli,86077Isernia,Italy` Correspondence should be addressed to Sarah Duse; [email protected] Received 9 February 2015; Accepted 10 May 2015 Academic Editor: John Byron Christoforidis Copyright © 2015 Francesco Semeraro et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The inner limiting membrane (ILM) is the basement membrane of theMuller¨ cells and can act as a scaffold for cellular proliferation in the pathophysiology of disorders affecting the vitreomacular interface. The atraumatic removal of the macular ILM has been proposed for treating various forms of tractional maculopathy in particular for macular pucker. In the last decade, the removal of ILM has become a routine practice in the surgery of the epiretinal membranes (ERMs), with good anatomical results. However many recent studies showed that ILM peeling is a procedure that can cause immediate traumatic effects and progressive modification on the underlying inner retinal layers. Moreover, it is unclear whether ILM peeling is helpful to improve vision after surgery for ERM. -

Physiology of the Retina
PHYSIOLOGY OF THE RETINA András M. Komáromy Michigan State University [email protected] 12th Biannual William Magrane Basic Science Course in Veterinary and Comparative Ophthalmology PHYSIOLOGY OF THE RETINA • INTRODUCTION • PHOTORECEPTORS • OTHER RETINAL NEURONS • NON-NEURONAL RETINAL CELLS • RETINAL BLOOD FLOW Retina ©Webvision Retina Retinal pigment epithelium (RPE) Photoreceptor segments Outer limiting membrane (OLM) Outer nuclear layer (ONL) Outer plexiform layer (OPL) Inner nuclear layer (INL) Inner plexiform layer (IPL) Ganglion cell layer Nerve fiber layer Inner limiting membrane (ILM) ©Webvision Inherited Retinal Degenerations • Retinitis pigmentosa (RP) – Approx. 1 in 3,500 people affected • Age-related macular degeneration (AMD) – 15 Mio people affected in U.S. www.nei.nih.gov Mutations Causing Retinal Disease http://www.sph.uth.tmc.edu/Retnet/ Retina Optical Coherence Tomography (OCT) Histology Monkey (Macaca fascicularis) fovea Ultrahigh-resolution OCT Drexler & Fujimoto 2008 9 Adaptive Optics Roorda & Williams 1999 6 Types of Retinal Neurons • Photoreceptor cells (rods, cones) • Horizontal cells • Bipolar cells • Amacrine cells • Interplexiform cells • Ganglion cells Signal Transmission 1st order SPECIES DIFFERENCES!! Photoreceptors Horizontal cells 2nd order Bipolar cells Amacrine cells 3rd order Retinal ganglion cells Visual Pathway lgn, lateral geniculate nucleus Changes in Membrane Potential Net positive charge out Net positive charge in PHYSIOLOGY OF THE RETINA • INTRODUCTION • PHOTORECEPTORS • OTHER RETINAL NEURONS -

組織學 Histology 台北醫學大學/解剖學科 教授:邱瑞珍 分機號碼:3261 電子郵件信箱:[email protected]
組織學 Histology 台北醫學大學/解剖學科 教授:邱瑞珍 分機號碼:3261 電子郵件信箱:[email protected] 1 Special sense – eye 台北醫學大學/解剖學科 馮琮涵 副教授 分機3250 E-mail: [email protected] 2 學習目的 •希望同學知道視網膜之顯微構造 •希望同學知道眼瞼顯微結構 3 參考資料 • Junqueira's Basic Histology, twelfth edition, text and atlas, Anthony L. Mescher, McGraw-Hill Companies 4 Summary • A) eye ball: – tunica fibrosa – cornea and sclera – tunica vasculosa—choroids, ciliary process, iris – tunica interna—retina – Lens – vitrous body • B) accessory structure: – eyelids – lacrimal gland 5 6 7 8 Eye • Wall of eyeball: tunicatunica fibrosafibrosa – cornea and sclera tunicatunica vasculosavasculosa – choroid, ciliary body & iris tunicatunica nervosanervosa – retina & pigmented layer opticoptic nervenerve & oraora serrataserrata • Contents: lens, aqueous humor & vitreous body 9 10 Cornea cornea – transparent, avascular, sensory innervation (CN V1) •• cornealcorneal epitheliumepithelium : non-cornified stratified squamous epi. •• BowmanBowman’’ss membranemembrane : basement membrane •• stromastroma : laminated collagen fibers; muco-polysaccharide matrix •• DescemetDescemet’’ss membranemembrane: basement membrane • endothelium 11 Cornea 12 Sclera sclera – thick c.t. with parallel collagen fibers, insertion site of extrinsic eye muscles • Epi-scleral tissue: loose vascular c.t., attaches conjunctiva • stroma: parallel collagen fibers Corneo-scleral junction (limbus): • trabecular meshwork, • canal of Schlemm (scleral venous sinus) 13 14 trabecular meshwork 15 Choroid choroid – soft and thin c.t. membrane • Supra-choroid layer : -

Origin and Turnover of ECM Proteins from the Inner Limiting Membrane
Eye (2008) 22, 1207–1213 & 2008 Macmillan Publishers Limited All rights reserved 0950-222X/08 $32.00 www.nature.com/eye 1 1 1 2 Origin and turnover W Halfter , S Dong , A Dong , AW Eller and CAMBRIDGE OPHTHALMOLOGY SYMPOSIUM R Nischt3 of ECM proteins from the inner limiting membrane and vitreous body Abstract ILM The inner limiting membrane (ILM) is a The inner limiting membrane (ILM) and the basement membrane (BM) that defines vitreous body (VB) are two major extracellular histologically the border between the retina and matrix (ECM) structures that are essential for the vitreous cavity. Functionally, the ILM is early eye development. The ILM is considered more appropriately considered as an adhesive to be the basement membrane of the retinal sheet that facilitates the connection of the neuroepithelium, yet in situ hybridization and vitreous body (VB) with the retina. Identical to chick/quail transplant experiments in organ- other BMs,1–3 the ILM consists of about ten cultured eyes showed that all components high-molecular weight extracellular matrix critical for ILM assembly, such as laminin or (ECM) proteins that include members of the collagen IV, are not synthesized by the retina. laminin family, nidogen1 and 2, collagen IV and Rather, ILM proteins, with the exception of three heparan sulphate proteoglycans, agrin, agrin, originate from the lens or (and) ciliary perlecan and collagen XVIII.4,5 The ILM is body and are shed into the vitreous. The VB 1 invisible by conventional light microscopy but Department of serves as a reservoir providing high Neurobiology, University of can be readily visualized by concentrations of ILM proteins for the instant Pittsburgh, Pittsburgh, PA, immunocytochemistry using antibodies to any USA assembly of new ILM during rapid embryonic of the BM components (Figure 1a). -
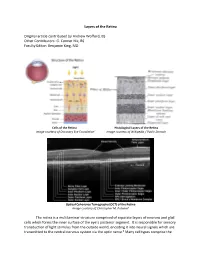
Layers of the Retina Original Article Contributed by Andrew Wofford, BS
Layers of the Retina Original article contributed by Andrew Wofford, BS Other Contributors: G. Conner Nix, BS Faculty Editor: Benjamin King, MD Cells of the Retina Histological Layers of the Retina Image courtesy of Discovery Eye Foundation1 Image courtesy of Wikipedia / Public Domain Optical Coherence Tomography (OCT) of the Retina Image courtesy of Christopher M. Putnam2 The retina is a multilaminar structure comprised of separate layers of neurons and glial cells which forms the inner surface of the eye’s posterior segment. It is responsible for sensory transduction of light stimulus from the outside world, encoding it into neural signals which are transmitted to the central nervous system via the optic nerve.3 Many cell types comprise the retina and allow it to perform its complex function; these include rods, cones, retinal ganglion cells, bipolar cells, Müller glial cells, horizontal cells, and amacrine cells.4 The ten layers of the retina from interior (bordering vitreous humor) to exterior (bordering choroid and sclera) are listed and described below.4,5 1. Inner Limiting Membrane – forms a barrier between the vitreous humor and the neurosensory retina. It is composed of the laterally branching foot plates of the Müller cells and is responsible for maintaining the structural integrity of the inner retina. • Clinical Correlation – The internal limiting membrane can be surgically removed for the treatment of several conditions, such as macular hole. 2. Nerve Fiber Layer – composed of ganglion cell axons that eventually converge to form the optic nerve. • Clinical Correlation – The nerve fiber layer and ganglion cell layers represent the part of the retina affected by glaucoma.