Evaluation of the National Guideline Clearinghouse (NGC)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Preliminary Catalogue of Isaac Roberts's Collection of Photographs of Celestial Objects
ASTRONOMISCHE NACHRICHTEN. Nr. 4154. Band 174. 2. Preliminary catalogue of Isaac Roberts’s collection of photographs of celestial objects. By Dorotha Isaac-Roberts. The Isaac Roberts collection of celestial photographs The number of the copies of the forthcoming paper comprises 2485 original negatives of stars, star-clusters, being very limited, the observatories and astronomers, official nebulae and other celestial objects together with many posi. or amateur, who are specially interested in photographic tives on glass and on paper, taken by the late Dr. Isaac astronomy will please send in their names to the address Roberts or, under his incessant supervision, by his assistant given below, early in 1907, in order that the various parts W. S. Franks, F. R. A. S., at Dr. Roberts’s Private Obser- of the complete catalogue may be sent to them in course vatory, Kennessee, Maghull, near Liverpool (1885-1 890) and, of time. from 1890 to 1904, at Starfield, Crowborough, Sussex. Positives- on-glass reproduced from the Isaac Roberts Of the above mentioned negatives, negatives will be lent for the purpose of micrometric mea- I) 1412 were taken with the zo-in Reflector (focal surements, if, application be made and provided that the length = 98.0 ins) and with an exposure of the plate of documents be returned after completion of the measurements. ninety minutes in general. In the preliminary catalogue given below, the numbers 2) 648 with the 5-in Cooke lens (f. 1. = 19.22 ins) of Dr. Dreyer’s New General Catalogue of Nebulae and which was mounted on the tube of the zo-in Reflector; or, Clusters of Stars and of Dr. -
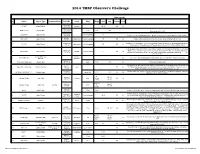
2014 Observers Challenge List
2014 TMSP Observer's Challenge Atlas page #s # Object Object Type Common Name RA, DEC Const Mag Mag.2 Size Sep. U2000 PSA 18h31m25s 1 IC 1287 Bright Nebula Scutum 20'.0 295 67 -10°47'45" 18h31m25s SAO 161569 Double Star 5.77 9.31 12.3” -10°47'45" Near center of IC 1287 18h33m28s NGC 6649 Open Cluster 8.9m Integrated 5' -10°24'10" Can be seen in 3/4d FOV with above. Brightest star is 13.2m. Approx 50 stars visible in Binos 18h28m 2 NGC 6633 Open Cluster Ophiuchus 4.6m integrated 27' 205 65 Visible in Binos and is about the size of a full Moon, brightest star is 7.6m +06°34' 17h46m18s 2x diameter of a full Moon. Try to view this cluster with your naked eye, binos, and a small scope. 3 IC 4665 Open Cluster Ophiuchus 4.2m Integrated 60' 203 65 +05º 43' Also check out “Tweedle-dee and Tweedle-dum to the east (IC 4756 and NGC 6633) A loose open cluster with a faint concentration of stars in a rich field, contains about 15-20 stars. 19h53m27s Brightest star is 9.8m, 5 stars 9-11m, remainder about 12-13m. This is a challenge obJect to 4 Harvard 20 Open Cluster Sagitta 7.7m integrated 6' 162 64 +18°19'12" improve your observation skills. Can you locate the miniature coathanger close by at 19h 37m 27s +19d? Constellation star Corona 5 Corona Borealis 55 Trace the 7 stars making up this constellation, observe and list the colors of each star asterism Borealis 15H 32' 55” Theta Corona Borealis Double Star 4.2m 6.6m .97” 55 Theta requires about 200x +31° 21' 32” The direction our Sun travels in our galaxy. -

Observing Galaxies in Pegasus 01 October 2015 23:07
Observing galaxies in Pegasus 01 October 2015 23:07 Context As you look towards Pegasus you are looking below the galactic plane under the Orion spiral arm of our galaxy. The Perseus-Pisces supercluster wall of galaxies runs through this constellation. It stretches from RA 3h +40 in Perseus to 23h +10 in Pegasus and is around 200 million light years away. It includes the Pegasus I group noted later this document. The constellation is well placed from mid summer to late autumn. Pegasus is a rich constellation for galaxy observing. I have observed 80 galaxies in this constellation. Relatively bright galaxies This section covers the galaxies that were visible with direct vision in my 16 inch or smaller scopes. This list will therefore grow over time as I have not yet viewed all the galaxies in good conditions at maximum altitude in my 16 inch scope! NGC 7331 MAG 9 This is the stand out galaxy of the constellation. It is similar to our milky way. Around it are a number of fainter NGC galaxies. I have seen the brightest one, NGC 7335 in my 10 inch scope with averted vision. I have seen NGC 7331 in my 25 x 100mm binoculars. NGC 7814 - Mag 10 ? Not on observed list ? This is a very lovely oval shaped galaxy. By constellation Page 1 NGC 7332 MAG 11 / NGC 7339 MAG 12 These galaxies are an isolated bound pair about 67 million light years away. NGC 7339 is the fainter of the two galaxies at the eyepiece. I have seen NGC 7332 in my 25 x 100mm binoculars. -

Cetus - the Whale
May 18 2021 Cetus - The Whale Observed: No Object Her Type Mag Alias/Notes IC 5384 Non-Existent NGC 7813 MCG -2-1-16 MK 936 IRAS 15-1215 PGC 287 IC 1528 Non-Existent NGC 7826 H29-8 Non-Existent Asterism IC 1533 Non-Existent NGC 34 Non-Existent NGC 17 NGC 58 Non-Existent NGC 47 PGC 967 MCG -1-1-55 IRAS 119-726 NGC 54 Glxy SB(r)a? 14.6 MCG -1-1-60 PGC 1011 NGC 59 Glxy SA(rs)0-: 13.1 ESO 539-4 MCG -4-1-26 PGC 1034 NGC 62 Glxy (R)SB(r)a: 12.3 MCG -2-1-43 IRAS 145-1345 PGC 1125 NGC 64 Glxy SB(s)bc 14 MCG -1-1-68 IRAS 149-706 PGC 1149 IC 5 Glxy E 14.8 MCG -2-1-47 IRAS 148-951 PGC 1145 NGC 73 Glxy SAB(rs)bc: 13.5 MCG -3-1-26 PGC 1211 NGC 65 Glxy SAB(rs)0-: 14.4 ESO 473-10A MCG -4-2-1 PGC 1229 NGC 66 Glxy SB(r)b pec 14.2 ESO 473-10 MCG -4-2-2 IRAS 165-2312 PGC 1236 IC 9 Glxy Sb(r) 16.1 MCG -2-2-1 IRAS 171-1423 PGC 1271 NGC 77 Glxy SA0-: 15.7 ESO 473-15 PGC 1290 NGC 102 Glxy S0/a 14.4 MCG -2-2-11 PGC 1542 NGC 107 Glxy Sbc 14.6 MCG -2-2-14 PGC 1606 NGC 111 Non-Existent NGC 113 Glxy SA0-: 13.5 MCG -1-2-16 PGC 1656 NGC 114 Glxy SB(rs)0: 14.7 UGC 259 MCG 0-2-27 MK 946 CGCG 383-14 KUG 24-20A PGC 1660 NGC 116 Non-Existent MCG -1-2-17 PGC 1671 NGC 117 Glxy S0+: sp 15.3 MCG 0-2-29 CGCG 383-15 PGC 1674 NGC 118 Glxy I0? 14.8 UGC 264 MCG 0-2-32 MK 947 CGCG 383-16 UM244 3ZW9 IRAS 247-203 PGC 1678 NGC 120 Glxy SB0^: 14.4 UGC 267 MCG 0-2-33 CGCG 383-17 PGC 1693 NGC 122 Non-Existent NGC 123 Non-Existent NGC 124 Glxy SA(s)c 13.7 UGC 271 MCG 0-2-38 CGCG 383-18 IRAS 253-205 PGC 1715 IC 15 Non-Existent IC 16 Glxy E? 14.7 MCG -2-2-17 IRAS 255-1322 PGC 1730 IC 17 -

Star Clusters in Local Group Galaxies 3
Massive Stellar Clusters ASP Conference Series, Vol. XX, 2000 A. Lan¸con & C. Boily, eds. Star Clusters in Local Group Galaxies – Impact of Environment on Their Evolution and Survival Eva K. Grebel1,2 1University of Washington, Department of Astronomy, Box 351580, Seattle, WA 98195, USA 2Hubble Fellow Abstract. Star clusters are found in ∼40% of the Local Group galax- ies. Their properties are reviewed. The impact of galaxy environment on the evolution and survival of star clusters is discussed. Possible evi- dence for cluster formation triggered by galaxy interactions, gradients in cluster size, metallicity and stellar content with galactocentric radius and variations as a function of galaxy type are briefly summarized. 1. Introduction Star clusters in Local Group galaxies comprise a wide range of roughly coeval stellar agglomerates ranging from globular clusters to associations, from very metal-poor objects to clusters with solar and higher metallicity, and from ancient populations to embedded young clusters. Three basic types of such clusterings are observed: globular clusters, open clusters, and associations. 1.1. Globular Clusters Globular clusters are centrally concentrated, spherical systems with masses of 4.2 ∼< log M[M⊙] ∼< 6.6 and tidal radii ranging from ∼ 10 to ∼ 100 pc. They are bound, long-lived (∼>10 Gyr) objects whose lifetimes may extend to a Hubble arXiv:astro-ph/9912529v2 30 Dec 1999 time or beyond, though due to various efficient destruction mechanisms the presently observed globulars are likely just a lower limit of the initial number of globular clusters. Globular clusters have been observed in all known types of galaxies, but many of the less massive galaxies do not contain globular clusters. -

Alma Observations of Warm Molecular Gas and Cold Dust in Ngc 34∗
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Caltech Authors The Astrophysical Journal, 787:48 (10pp), 2014 May 20 doi:10.1088/0004-637X/787/1/48 C 2014. The American Astronomical Society. All rights reserved. Printed in the U.S.A. ALMA OBSERVATIONS OF WARM MOLECULAR GAS AND COLD DUST IN NGC 34∗ C. K. Xu1,C.Cao1,2,3,N.Lu1,Y.Gao4, P. van der Werf5,A.S.Evans6,7, J. M. Mazzarella1, J. Chu8,S.Haan9, T. Diaz-Santos1, R. Meijerink5, Y.-H. Zhao1,4, P. Appleton1, L. Armus1, V. Charmandaris10,11,12,S.Lord1, E. J. Murphy1, D. B. Sanders8, B. Schulz1, and S. Stierwalt6,13 1 Infrared Processing and Analysis Center, MS 100-22, California Institute of Technology, Pasadena, CA 91125, USA 2 School of Space Science and Physics, Shandong University at Weihai, Weihai, Shandong 264209, China; [email protected] 3 Shandong Provincial Key Laboratory of Optical Astronomy and Solar-Terrestrial Environment, Weihai, Shandong 264209, China 4 Purple Mountain Observatory, Chinese Academy of Sciences, 2 West Beijing Road, Nanjing 210008, China 5 Leiden Observatory, Leiden University, P.O. Box 9513, NL-2300 RA Leiden, Netherlands 6 Department of Astronomy, University of Virginia, P.O. Box 400325, Charlottesville, VA 22904, USA 7 National Radio Astronomy Observatory, Charlottesville, VA 22904, USA 8 Institute for Astronomy, University of Hawaii, 2680 Woodlawn Drive, Honolulu, HI 96816, USA 9 CSIRO Astronomy and Space Science, ATNF, P.O. Box 76, Epping 1710, Australia 10 Department of Physics, University of Crete, GR-71003, Heraklion, Greece 11 Institute for Astronomy, Astrophysics, Space Applications & Remote Sensing, National Observatory of Athens, GR-15236, Penteli, Greece 12 Chercheur Associe,´ Observatoire de Paris, F-75014, Paris, France 13 Spitzer Science Center, California Institute of Technology, 1200 E. -

7.5 X 11.5.Threelines.P65
Cambridge University Press 978-0-521-19267-5 - Observing and Cataloguing Nebulae and Star Clusters: From Herschel to Dreyer’s New General Catalogue Wolfgang Steinicke Index More information Name index The dates of birth and death, if available, for all 545 people (astronomers, telescope makers etc.) listed here are given. The data are mainly taken from the standard work Biographischer Index der Astronomie (Dick, Brüggenthies 2005). Some information has been added by the author (this especially concerns living twentieth-century astronomers). Members of the families of Dreyer, Lord Rosse and other astronomers (as mentioned in the text) are not listed. For obituaries see the references; compare also the compilations presented by Newcomb–Engelmann (Kempf 1911), Mädler (1873), Bode (1813) and Rudolf Wolf (1890). Markings: bold = portrait; underline = short biography. Abbe, Cleveland (1838–1916), 222–23, As-Sufi, Abd-al-Rahman (903–986), 164, 183, 229, 256, 271, 295, 338–42, 466 15–16, 167, 441–42, 446, 449–50, 455, 344, 346, 348, 360, 364, 367, 369, 393, Abell, George Ogden (1927–1983), 47, 475, 516 395, 395, 396–404, 406, 410, 415, 248 Austin, Edward P. (1843–1906), 6, 82, 423–24, 436, 441, 446, 448, 450, 455, Abbott, Francis Preserved (1799–1883), 335, 337, 446, 450 458–59, 461–63, 470, 477, 481, 483, 517–19 Auwers, Georg Friedrich Julius Arthur v. 505–11, 513–14, 517, 520, 526, 533, Abney, William (1843–1920), 360 (1838–1915), 7, 10, 12, 14–15, 26–27, 540–42, 548–61 Adams, John Couch (1819–1892), 122, 47, 50–51, 61, 65, 68–69, 88, 92–93, -

Kinematics of the Local Universe
ASTRONOMY & ASTROPHYSICS JUNE I 1998, PAGE 333 SUPPLEMENT SERIES Astron. Astrophys. Suppl. Ser. 130, 333–339 (1998) Kinematics of the local universe VII. New 21-cm line measurements of 2112 galaxies?,?? G. Theureau1, L. Bottinelli1,3, N. Coudreau-Durand1, L. Gouguenheim1,3, N. Hallet1, M. Loulergue1, G. Paturel4, and P. Teerikorpi2 1 Observatoire de Paris/Meudon, ARPEGES/CNRS URA1757, F-92195 Meudon Principal Cedex, France 2 Tuorla Observatory, 21500 Piikki¨o, Finland 3 Universit´e Paris-Sud, F-91405 Orsay, France 4 Observatoire de Lyon, F-69561 Saint-Genis Laval Cedex, France Received November 28; accepted December 24, 1997 Abstract. This paper presents 2112 new 21-cm neutral hy- • the completeness of the sample (Paturel et al. 1994) drogen line measurements carried out with the meridian • the homogenization of optical and radio data in con- transit Nan¸cay radiotelescope. Among these data we give nexion with the Lyon-Meudon Extragalactic database also 213 new radial velocities which complement those LEDA (Bottinelli et al. 1990) listed in three previous papers of this series. These new • the effect of disc opaqueness on observed optical B- measurements, together with the HI data collected in band parameters (Bottinelli et al. 1995) LEDA, put to 6 700 the number of galaxies with 21-cm • the dependence of the TF relation on morphological line width, radial velocity, and apparent diameter in the type and mean surface brightness and the improve- so-called KLUN sample. ment of this distance indicator (Theureau et al. 1997a; Theureau 1998) Key words: catalogs — galaxies: distances and redshifts; • the determination of the Hubble constant H0 from TF ISM — radio lines: galaxies B-band and log D25 relations (Theureau et al. -
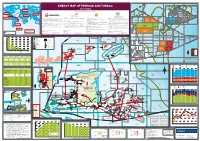
View the Energy Map of Trinidad and Tobago, 2017 Edition
Trinidad and Tobago LNG export destinations 2015 (million m3 of LNG) Trinidad and Tobago deepwater area for development 60°W 59°W 58°W Trinidad and Tobago territorial waters ENERGY MAP OF TRINIDAD AND TOBAGO 1000 2000 m m 2017 edition GRENADA BARBADOS Trinidad and Tobago 2000m LNG to Europe A tlantic Ocean 2.91 million m3 LNG A TLANTIC Caribbean 2000m 20 Produced by Petroleum Economist, in association with 00 O CEAN EUROPE Sea m Trinidad and Tobago 2000m LNG to North America NORTH AMERICA Trinidad and Tobago 5.69 million m3 LNG LNG to Asia 3 GO TTDAA 30 TTDAA 31 TTDAA 32 BA 1.02 million m LNG TO Trinidad and Tobago OPEN OPEN OPEN 12°N LNG to MENA 3 1.90 million m LNG ASIA Trinidad and Tobago CARIBBEAN TRINIDAD AND TOBAGO 2000m LNG to Caribbean Atlantic LNG Company profile Company Profile Company profile Company Profile 3 Established by the Government of Trinidad and Tobago in August 1975, The National Gas Company of Trinidad and Tobago Limited (NGC) is an BHP Billiton is a leading global resources company with a Petroleum Business that includes exploration, development, production and marketing Shell has been in Trinidad and Tobago for over 100 years and has played a major role in the development of its oil and gas industry. Petroleum Company of Trinidad and Tobago Limited (Petrotrin) is an integrated Oil and Gas Company engaged in the full range of petroleum 3.84 million m LNG internationally investment-graded company that is strategically positioned in the midstream of the natural gas value chain. -

Making a Sky Atlas
Appendix A Making a Sky Atlas Although a number of very advanced sky atlases are now available in print, none is likely to be ideal for any given task. Published atlases will probably have too few or too many guide stars, too few or too many deep-sky objects plotted in them, wrong- size charts, etc. I found that with MegaStar I could design and make, specifically for my survey, a “just right” personalized atlas. My atlas consists of 108 charts, each about twenty square degrees in size, with guide stars down to magnitude 8.9. I used only the northernmost 78 charts, since I observed the sky only down to –35°. On the charts I plotted only the objects I wanted to observe. In addition I made enlargements of small, overcrowded areas (“quad charts”) as well as separate large-scale charts for the Virgo Galaxy Cluster, the latter with guide stars down to magnitude 11.4. I put the charts in plastic sheet protectors in a three-ring binder, taking them out and plac- ing them on my telescope mount’s clipboard as needed. To find an object I would use the 35 mm finder (except in the Virgo Cluster, where I used the 60 mm as the finder) to point the ensemble of telescopes at the indicated spot among the guide stars. If the object was not seen in the 35 mm, as it usually was not, I would then look in the larger telescopes. If the object was not immediately visible even in the primary telescope – a not uncommon occur- rence due to inexact initial pointing – I would then scan around for it. -

Ngc Catalogue Ngc Catalogue
NGC CATALOGUE NGC CATALOGUE 1 NGC CATALOGUE Object # Common Name Type Constellation Magnitude RA Dec NGC 1 - Galaxy Pegasus 12.9 00:07:16 27:42:32 NGC 2 - Galaxy Pegasus 14.2 00:07:17 27:40:43 NGC 3 - Galaxy Pisces 13.3 00:07:17 08:18:05 NGC 4 - Galaxy Pisces 15.8 00:07:24 08:22:26 NGC 5 - Galaxy Andromeda 13.3 00:07:49 35:21:46 NGC 6 NGC 20 Galaxy Andromeda 13.1 00:09:33 33:18:32 NGC 7 - Galaxy Sculptor 13.9 00:08:21 -29:54:59 NGC 8 - Double Star Pegasus - 00:08:45 23:50:19 NGC 9 - Galaxy Pegasus 13.5 00:08:54 23:49:04 NGC 10 - Galaxy Sculptor 12.5 00:08:34 -33:51:28 NGC 11 - Galaxy Andromeda 13.7 00:08:42 37:26:53 NGC 12 - Galaxy Pisces 13.1 00:08:45 04:36:44 NGC 13 - Galaxy Andromeda 13.2 00:08:48 33:25:59 NGC 14 - Galaxy Pegasus 12.1 00:08:46 15:48:57 NGC 15 - Galaxy Pegasus 13.8 00:09:02 21:37:30 NGC 16 - Galaxy Pegasus 12.0 00:09:04 27:43:48 NGC 17 NGC 34 Galaxy Cetus 14.4 00:11:07 -12:06:28 NGC 18 - Double Star Pegasus - 00:09:23 27:43:56 NGC 19 - Galaxy Andromeda 13.3 00:10:41 32:58:58 NGC 20 See NGC 6 Galaxy Andromeda 13.1 00:09:33 33:18:32 NGC 21 NGC 29 Galaxy Andromeda 12.7 00:10:47 33:21:07 NGC 22 - Galaxy Pegasus 13.6 00:09:48 27:49:58 NGC 23 - Galaxy Pegasus 12.0 00:09:53 25:55:26 NGC 24 - Galaxy Sculptor 11.6 00:09:56 -24:57:52 NGC 25 - Galaxy Phoenix 13.0 00:09:59 -57:01:13 NGC 26 - Galaxy Pegasus 12.9 00:10:26 25:49:56 NGC 27 - Galaxy Andromeda 13.5 00:10:33 28:59:49 NGC 28 - Galaxy Phoenix 13.8 00:10:25 -56:59:20 NGC 29 See NGC 21 Galaxy Andromeda 12.7 00:10:47 33:21:07 NGC 30 - Double Star Pegasus - 00:10:51 21:58:39 -

WAVES of EXTRATERRESTRIAL ORIGIN DISSERTATION Presented
AN INVESTIGATION AND ANALYSIS OF RATIO ' WAVES OF EXTRATERRESTRIAL ORIGIN DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By HSIEN-CHING KO, B.S., M.S. The Ohio State University 1 9 # Approved by: _ Adviser Department of Electrical Engineering ACKNOWLEDGEMENTS The research presented in this dissertation was done at the Radio Observatory of The Ohio State University under the supervision of Professor John D* Kraus who originated the radio astronory project, designed the radio telescope and guided the research. The author wishes to express his sincere appreciation to Professor Kraus, his adviser, for his helpful guidance, discussion and review of the manuscript. For three years he has continuously- provided the author with every assistance possible in order that the present investigation could be successfully completed. Dr. Kraus has contributed many valuable new ideas throughout the investigation* It is a pleasure to acknowledge the work of Mr. Dorm Van Stoutenburg in connection with the improvement and maintenance of the equipment. Thanks are also due many others who participated in the construction of the radio telescope. In addition, rry thanks go to Miss Pi-Yu Chang and Miss Justine Wilson for their assistance in the preparation of the manuscript, and to Mr. Charles E. Machovec of the Physics Library for his cooperation in using the reference materials• The radio astronony project is supported ty grants from the Development Fund,