Plant Community Development of Isle Royale's Moose-Spruce Savannas Michael C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix 2: Plant Lists
Appendix 2: Plant Lists Master List and Section Lists Mahlon Dickerson Reservation Botanical Survey and Stewardship Assessment Wild Ridge Plants, LLC 2015 2015 MASTER PLANT LIST MAHLON DICKERSON RESERVATION SCIENTIFIC NAME NATIVENESS S-RANK CC PLANT HABIT # OF SECTIONS Acalypha rhomboidea Native 1 Forb 9 Acer palmatum Invasive 0 Tree 1 Acer pensylvanicum Native 7 Tree 2 Acer platanoides Invasive 0 Tree 4 Acer rubrum Native 3 Tree 27 Acer saccharum Native 5 Tree 24 Achillea millefolium Native 0 Forb 18 Acorus calamus Alien 0 Forb 1 Actaea pachypoda Native 5 Forb 10 Adiantum pedatum Native 7 Fern 7 Ageratina altissima v. altissima Native 3 Forb 23 Agrimonia gryposepala Native 4 Forb 4 Agrostis canina Alien 0 Graminoid 2 Agrostis gigantea Alien 0 Graminoid 8 Agrostis hyemalis Native 2 Graminoid 3 Agrostis perennans Native 5 Graminoid 18 Agrostis stolonifera Invasive 0 Graminoid 3 Ailanthus altissima Invasive 0 Tree 8 Ajuga reptans Invasive 0 Forb 3 Alisma subcordatum Native 3 Forb 3 Alliaria petiolata Invasive 0 Forb 17 Allium tricoccum Native 8 Forb 3 Allium vineale Alien 0 Forb 2 Alnus incana ssp rugosa Native 6 Shrub 5 Alnus serrulata Native 4 Shrub 3 Ambrosia artemisiifolia Native 0 Forb 14 Amelanchier arborea Native 7 Tree 26 Amphicarpaea bracteata Native 4 Vine, herbaceous 18 2015 MASTER PLANT LIST MAHLON DICKERSON RESERVATION SCIENTIFIC NAME NATIVENESS S-RANK CC PLANT HABIT # OF SECTIONS Anagallis arvensis Alien 0 Forb 4 Anaphalis margaritacea Native 2 Forb 3 Andropogon gerardii Native 4 Graminoid 1 Andropogon virginicus Native 2 Graminoid 1 Anemone americana Native 9 Forb 6 Anemone quinquefolia Native 7 Forb 13 Anemone virginiana Native 4 Forb 5 Antennaria neglecta Native 2 Forb 2 Antennaria neodioica ssp. -

(Betula Papyrifera) / Diervilla Lonicera Trembling Aspen (Paper Birch) / Northern Bush-Honeysuckle Peuplier Faux-Tremble (Bouleau À Papier) / Dièreville Chèvrefeuille
Forest / Forêt Association CNVC 00238 Populus tremuloides (Betula papyrifera) / Diervilla lonicera Trembling Aspen (Paper Birch) / Northern Bush-honeysuckle Peuplier faux-tremble (Bouleau à papier) / Dièreville chèvrefeuille Subassociations: 238a typic, 238b Alnus viridis, 238c Kalmia angustifolia CNVC Alliance: CA00014 Betula papyrifera – Populus tremuloides – Abies balsamea / Clintonia borealis CNVC Group: CG0007 Ontario-Quebec Boreal Mesic Paper Birch – Balsam Fir – Trembling Aspen Forest Type Description Concept: CNVC00238 is a boreal hardwood forest Association that ranges from Manitoba to Quebec. It has a closed canopy dominated by trembling aspen ( Populus tremuloides ), usually with paper birch ( Betula papyrifera ), overtopping a well-developed to dense shrub layer. The shrub layer includes a mix of regenerating tree species, primarily trembling aspen, balsam fir ( Abies balsamea ), paper birch and black spruce ( Picea mariana ), as well as low shrub species, such as velvet-leaved blueberry ( Vaccinium myrtilloides ), northern bush-honeysuckle ( Diervilla lonicera ) and early lowbush blueberry ( V. angustifolium ). The herb layer is well developed and typically includes bunchberry (Cornus canadensis ), wild lily-of-the-valley ( Maianthemum canadense ), wild sarsaparilla ( Aralia nudicaulis ), yellow clintonia ( Clintonia borealis ), twinflower ( Linnaea borealis ) and northern starflower Source: Natural Resources Canada - (Lysimachia borealis ). The forest floor cover is mainly broad-leaf litter so the moss layer is Canadian Forest Service sparse, with only minor cover of red-stemmed feathermoss ( Pleurozium schreberi) . CNVC00238 is an early seral condition that typically establishes after fire or harvesting. It occurs in a region with a continental boreal climate that grades from subhumid in the west to humid in the east and is usually found on mesic, nutrient-medium sites. -

And Natural Community Restoration
RECOMMENDATIONS FOR LANDSCAPING AND NATURAL COMMUNITY RESTORATION Natural Heritage Conservation Program Wisconsin Department of Natural Resources P.O. Box 7921, Madison, WI 53707 August 2016, PUB-NH-936 Visit us online at dnr.wi.gov search “ER” Table of Contents Title ..……………………………………………………….……......………..… 1 Southern Forests on Dry Soils ...................................................... 22 - 24 Table of Contents ...……………………………………….….....………...….. 2 Core Species .............................................................................. 22 Background and How to Use the Plant Lists ………….……..………….….. 3 Satellite Species ......................................................................... 23 Plant List and Natural Community Descriptions .…………...…………….... 4 Shrub and Additional Satellite Species ....................................... 24 Glossary ..................................................................................................... 5 Tree Species ............................................................................... 24 Key to Symbols, Soil Texture and Moisture Figures .................................. 6 Northern Forests on Rich Soils ..................................................... 25 - 27 Prairies on Rich Soils ………………………………….…..….……....... 7 - 9 Core Species .............................................................................. 25 Core Species ...……………………………….…..…….………........ 7 Satellite Species ......................................................................... 26 Satellite Species -

Pollinator-Friendly Plants for the Northeast United States
Pollinator-Friendly Plants for the Northeast United States for more information on pollinators and outreach, please click here Information provided in the following document was obtained from a variety of sources and field research conducted at the USDA NRCS Big Flats Plant Materials Center. Sources of information can be found by clicking here. Pollinator rating values were provided by the Xerces Society as well as past and current research. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or a part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, D.C. 20250- 9410 or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer. USDA is committed to making its information materials accessible to all USDA customers and employees. Table of Contents Click on species to jump to that page • Purple Giant Hyssop • -

A Comparative Study of Cultivated Asters Richard G
Plant Evaluation Notes ISSUE 36, 2013 A Comparative Study of Cultivated Asters Richard G. Hawke, Plant Evaluation Manager Jessie Vining Stevens Symphyotrichum oblongifolium ‘Raydon’s Favorite’ utumn is the time of asters. In days one of the largest and most evolutionarily sion, white. The ray florets surround the clus- suffused with the brilliant tones of specialized of plant families. The familial re- ter of disk florets; the number of rays varies senescing leaves, asters finally show semblance is evident among aster relatives from a few to hundreds in some double-flow- their true colors in gardens, both cultivated such as dahlias (Dahlia spp.), coneflowers ered cultivars. Each ray floret has one long, and natural, along roadsides, and in native (Echinacea spp.), sunflowers (Helianthus narrow ligule that is distinctly petallike in ap- places. Like clockwork, their starry flowers in spp.), Shasta daisies (Leucanthemum spp.), pearance, and acts much like the petal of a rich hues of blue, purple, pink, or white burst and zinnias (Zinnia spp.). Recently, changes in typical flower to attract pollinators to the forth to mark the change of seasons. A ubiq- the generic names of North American species plant. Ray florets come in varying shades of uitous nature often saddles asters with the from Aster to less melodious names such as pink, red, lavender, blue, violet, purple, and reputation of looking too wild, but their natu- Doellingeria, Eurybia, and Symphyotrichum white; the rays rather than the disks describe ral beauty and garden merit cannot be over- have complicated matters for gardeners. The the overall flower color. Another attribute of looked. -

White Wood Aster (Eurybia Divaricata) in Ontario
Photo: Rob Tervo White Wood Aster (Eurybia divaricata) in Ontario Ontario Recovery Strategy Series 2019 Ministry of the Environment, Conservation and Parks About the Ontario Recovery Strategy Series This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada. What is recovery? What’s next? Recovery of species at risk is the process by Nine months after the completion of a recovery which the decline of an endangered, threatened, strategy a government response statement will or extirpated species is arrested or reversed, be published which summarizes the actions that and threats are removed or reduced to improve the Government of Ontario intends to take in the likelihood of a species’ persistence in the response to the strategy. The implementation of wild. recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and What is a recovery strategy? conservationists. Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A For more information recovery strategy outlines the habitat needs To learn more about species at risk recovery in and the threats to the survival and recovery of Ontario, please visit the Ministry of Environment, the species. It also makes recommendations Conservation and Parks Species at Risk webpage on the objectives for protection and recovery, at: www.ontario.ca/speciesatrisk the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. -

Aster À Rameaux Étalés (Eurybia Divaricata) Au Canada
Loi sur les espèces en péril Série de Programmes de rétablissement Programme de rétablissement de l’aster à rameaux étalés (Eurybia divaricata) au Canada Aster à rameaux étalés 2018 Référence recommandée : Environnement et Changement climatique Canada. 2018. Programme de rétablissement de l’aster à rameaux étalés (Eurybia divaricata) au Canada, Série de Programmes de rétablissement de la Loi sur les espèces en péril, Environnement et Changement climatique Canada, Ottawa, viii + 71 p. Pour télécharger le présent programme de rétablissement ou pour obtenir un complément d’information sur les espèces en péril, incluant les rapports de situation du Comité sur la situation des espèces en péril au Canada (COSEPAC), les descriptions de la résidence, les plans d’action et d’autres documents connexes portant sur le rétablissement, veuillez consulter le Registre public des espèces en péril1. Illustration de la couverture : © Vanessa Dufresne, Environnement et Changement climatique Canada, Service canadien de la faune – Région du Québec Also available in English under the title “Recovery Strategy for the White Wood Aster (Eurybia divaricata) in Canada” © Sa Majesté la Reine du chef du Canada, représentée par la ministre de l’Environnement et du Changement climatique, 2018. Tous droits réservés. ISBN 978-0-660-28931-1 No de catalogue En3-4/307-2018F-PDF Le contenu du présent document (à l’exception des illustrations) peut être utilisé sans permission, mais en prenant soin d’indiquer la source. 1 http://sararegistry.gc.ca/default.asp?lang=Fr&n=24F7211B-1 Programme de rétablissement de l’aster à rameaux étalés 2018 1 Préface 2 3 En vertu de l’Accord pour la protection des espèces en péril (1996)2, les gouvernements 4 fédéral, provinciaux et territoriaux signataires ont convenu d’établir une législation et 5 des programmes complémentaires qui assureront la protection efficace des espèces en 6 péril partout au Canada. -

High Line Plant List Stay Connected @Highlinenyc
BROUGHT TO YOU BY HIGH LINE PLANT LIST STAY CONNECTED @HIGHLINENYC Trees & Shrubs Acer triflorum three-flowered maple Indigofera amblyantha pink-flowered indigo Aesculus parviflora bottlebrush buckeye Indigofera heterantha Himalayan indigo Amelanchier arborea common serviceberry Juniperus virginiana ‘Corcorcor’ Emerald Sentinel® eastern red cedar Amelanchier laevis Allegheny serviceberry Emerald Sentinel ™ Amorpha canescens leadplant Lespedeza thunbergii ‘Gibraltar’ Gibraltar bushclover Amorpha fruticosa desert false indigo Magnolia macrophylla bigleaf magnolia Aronia melanocarpa ‘Viking’ Viking black chokeberry Magnolia tripetala umbrella tree Betula nigra river birch Magnolia virginiana var. australis Green Shadow sweetbay magnolia Betula populifolia grey birch ‘Green Shadow’ Betula populifolia ‘Whitespire’ Whitespire grey birch Mahonia x media ‘Winter Sun’ Winter Sun mahonia Callicarpa dichotoma beautyberry Malus domestica ‘Golden Russet’ Golden Russet apple Calycanthus floridus sweetshrub Malus floribunda crabapple Calycanthus floridus ‘Michael Lindsey’ Michael Lindsey sweetshrub Nyssa sylvatica black gum Carpinus betulus ‘Fastigiata’ upright European hornbeam Nyssa sylvatica ‘Wildfire’ Wildfire black gum Carpinus caroliniana American hornbeam Philadelphus ‘Natchez’ Natchez sweet mock orange Cercis canadensis eastern redbud Populus tremuloides quaking aspen Cercis canadensis ‘Ace of Hearts’ Ace of Hearts redbud Prunus virginiana chokecherry Cercis canadensis ‘Appalachian Red’ Appalachian Red redbud Ptelea trifoliata hoptree Cercis -

Asteraceae – Aster Family
ASTERACEAE – ASTER FAMILY Plant: herbs (annual or perennial), some shrubs, rarely vines or trees. Stem: Root: Often with tubers, rhizomes, stolons, or fleshy roots Leaves: mostly simple, some compound, alternate or opposite, rarely whorled. Flowers: flower head supported by an involucre (whorl of green bracts or phyllaries); each head composed of small flowers (composite) of flat ray-like (ligulate) flowers on the outside (ray flowers) and central tube-like flowers (disk flowers) – some species may have only one or the other. Calyx absent or modified into hairs, bristles, scales or a crown (pappus); 5 stamens (syngenesious -united by anthers); 5 united petals (sympetalous), receptacle may also have hairs or bristles. Both pappus and receptacle hairs/bristles may be used in ID. Fruit: achene (small, one-seeded, inferior ovule, 2 carpels, hard shell fruit) often with persisting crowned pappus which helps with seed dispersal. Other: Very large family, divided into sub-families and tribes, once named Compositae; 1-2,000 genera, 20,000+ species. Dicotyledons Group WARNING – family descriptions are only a layman’s guide and should not be used as definitive ASTERACEAE – ASTER FAMILY Flat-Topped White Aster [Parasol Whitetop]; Doellingeria umbellata (P. Mill.) Nees var. umbellata Fetid Marigold; Dyssodia papposa (Vent.) A.S. Hitchc. Yellow Coneflower; Echinacea paradoxa (J.B.S. Norton) Britton var. paradoxa [Eastern] Purple Cone-Flower; Echinacea purpurea (L.) Moench [Glade or Pale Purple] Coneflower; Echinacea simulata R.L. McGregor OR Echinacea pallida (Nutt.) Nutt. False Daisy [Yerba De Tajo]; Eclipta prostrata (L.) L. (Eclipta alba) Carolina Elephant’s-Foot; Elephantopus carolinianus Raeusch. Devil's Grandmother; Elephantopus tomentosus L. -

Botanical Survey for Regional Forester Sensitive Species
Botanical Survey for Regional Forester Sensitive Species Mesabi Black Quarry Grout Pile Expansion Lake County, Minnesota SEH No. CSGCO 120845 November 2012 Mesabi Black Quarry Grout Pile Expansion Botanical Survey for Regional Forester Sensitive Species Lake County, Minnesota SEH No. CSGCO 120845 November 2012 For Review and Analysis in Support of and Inclusion in a Federal Environmental Assessment Prepared by the U.S. Forest Service U.S. Forest Service Superior National Forest 8901 Grand Avenue Place Duluth, MN 55808 I hereby certify that this report was prepared by me or under my direct supervision. I hereby certify that this report was prepared by me or under my direct supervision. Prepared by: Natalie White, MS, CWD | Biologist MN Certified Wetland Delineator, No. 1226 Reviewed by: Allyz Kramer, MS, PWS, CWD | Sr. Biologist, Project Manager MN Certified Wetland Delineator, No. 1023 Professional Wetland Scientist, No. 1884 MNDNR Approved Botanical Surveyor Short Elliott Hendrickson Inc. 418 West Superior Street Duluth, MN 55802-1512 218.279.3000 Table of Contents Letter of Transmittal Certification Page Table of Contents Page 1.0 Introduction ................................................................................................................1 2.0 Site Location ..............................................................................................................1 3.0 Survey Methodology .................................................................................................1 3.1.1 Background Investigation -

White Pine Driftless Bluff Forest
White Pine Driftless Bluff Forest Site Characteristics: This community is a relict, thought to have existed in the Driftless Area since at least the Wisconsin glaciation. This dry-mesic to mesic pine bluff forest occurs on steep sandstone or, rarely, limestone bluffs in the Driftless Area of the upper Midwestern United States. Stands occur in small patches 0.1-2 ha in size on soils derived of glacial till or outwash plains. The soils are shallow, poorly developed, often acidic and excessively drained. Soils are typically loamy sands, sandy loams, loams, or clay loams with low organic material. Runoff and leaching are severe due to the slope and the porous nature of the bedrock. This community occurs on sloping or level sandstone or, rarely, on quartzite bluffs. The sandstone is either St. Peter or Franconia. McIntosh (1950) studied 22 stands of this type and found them on all aspects except SE. This community is most commonly found on water worn cliffs with an undercutting stream at the cliff base. Considerable light can penetrate to the forest floor even though the trees are close together. Vegetation Characteristics: This community has a closed to open canopy of conifer or mixed conifer-hardwood trees, with White Pine as the dominant species. The composition of the understory is variable but typically contains herbs and shrubs with some prairie species. The shrub layer is absent to well-developed, while the herbaceous layer is moderately to poorly developed (Sims et al. 1989). Understory vegetation is sparse where the canopy is closed due to the limited amount of light and duff buildup on the forest floor. -
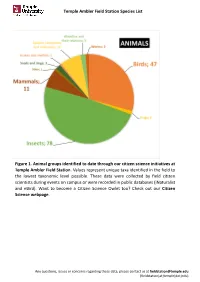
Temple Ambler Field Station Species List Figure 1. Animal Groups Identified to Date Through Our Citizen Science Initiatives at T
Temple Ambler Field Station Species List Figure 1. Animal groups identified to date through our citizen science initiatives at Temple Ambler Field Station. Values represent unique taxa identified in the field to the lowest taxonomic level possible. These data were collected by field citizen scientists during events on campus or were recorded in public databases (iNaturalist and eBird). Want to become a Citizen Science Owlet too? Check out our Citizen Science webpage. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List Figure 2. Plant diversity identified to date in the natural environments and designed gardens of the Temple Ambler Field Station and Ambler Arboretum. These values represent unique taxa identified to the lowest taxonomic level possible. Highlighted are 14 of the 116 flowering plant families present that include 524 taxonomic groups. A full list can be found in our species database. Cultivated specimens in our Greenhouse were not included here. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List database_title Temple Ambler Field Station Species List last_update 22October2020 description This database includes all species identified to their lowest taxonomic level possible in the natural environments and designed gardens on the Temple Ambler campus. These are occurrence records and each taxon is only entered once. This is an occurrence record, not an abundance record. IDs were performed by senior scientists and specialists, as well as citizen scientists visiting campus.