Antill Tim Spring 2010
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fording River Operations: the Swift Project Description
Fording River Operations: The Swift Project Description Submitted to: BC Environmental Assessment Office 1st Floor 836 Yates Street PO Box 9426 Stn Prov Govt Victoria, BC, V8W 9V1 Pursuant to: British Columbia Environmental Assessment Act Submitted by: Teck Coal Limited – Fording River Operations April 2011 Teck Coal Limited – Fording River Operations: The Swift Project TABLE OF CONTENTS 1 PROPONENT INFORMATION ...................................................................... 1 1.1 Key Proponent Contacts .................................................................................................. 1 2 PROJECT INFORMATION ............................................................................ 2 2.1 Location ........................................................................................................................... 2 2.2 Project History and Overview .......................................................................................... 2 2.3 Deposit Geology and Resource Characterization ........................................................... 6 2.3.1 Stratigraphy ......................................................................................................... 6 2.3.2 Structure .............................................................................................................. 8 2.4 Components and Infrastructure ..................................................................................... 10 2.4.1 Conceptual Mine Plan and Schedule ............................................................... -

Castle Project Initial Project Description in Accordance with Schedule 1 of the Impact Assessment Act Information and Management of Time Limits Regulations
Castle Project Initial Project Description in accordance with Schedule 1 of the Impact Assessment Act Information and Management of Time Limits Regulations October 2020 Teck Coal Limited Fording River Operations P.O. Box 100 +1 250 865 2271 Tel Elkford, B.C. Canada V0B 1H0 www.teck.com October 9, 2020 Fraser Ross Project Manager Impact Assessment Agency of Canada 210A - 757 West Hastings Street Vancouver, BC, V6C 3M2 Dear Mr. Ross Reference: Fording River Operations Castle Initial Project Description As requested by the Impact Assessment Agency of Canada (IAAC, the Agency), Teck Coal Limited is submitting the attached 2-part document to satisfy the federal requirements of an Initial Project Description (IPD) for the Fording River Operations Castle Project: 1. Provincial IPD published in April 2020 - The provincial IPD was previously submitted to the British Columbia (BC) Environmental Assessment Office in April 2020 and was prepared to satisfy information requirements under the BC Environmental Assessment Act. 1. IPD Addendum – the IPD Addendum focuses on providing supplemental information required by the Agency to satisfy the requirements of an IPD in accordance with Schedule 1 of the Information and Management of Time Limits Regulations under the Impact Assessment Act of Canada. Summaries of the IPD documents noted above, in English and in French, are provided under separate cover. Please contact the undersigned if you have any questions or comments on the enclosed material. Sincerely, David Baines Senior Lead Regulatory Approvals Teck Coal Limited Initial Project Description: Castle Project Teck Coal Limited Fording River Operations April 2020 Initial Project Description: Castle Project Executive Summary Introduction This document is an Initial Project Description (IPD) for the Teck Coal Limited (Teck) Fording River Operations Castle Project (the Castle Project or the Project) under the British Columbia (BC) Environmental Assessment Act (BC EAA) (SBC 2018, c 51). -

The Pinnacle Club Journal 1950 '—
THE © PinnaclePINNACLE Club and Author All Rights Reserved CLUB JOURNAL I95O Edited by HELEN BRYAN CONTENTS Page Foreword E. Wells .. 7 Eighteen Days in the Western Himalayas.. Gladys Jean Low (Mrs. Ptame/t) 9 Alpine Meet, 1949 E. Worsley Leech 21 Reminiscences of the Canadian Rockies .. M.I.Jeffrey .. 26 Bosigran Nea Morin 33 Munro-ing A. Hirst Laliderer Spitze, Nordkanta E. Longstaff Two Visits to Arran E. Wells .. 51 Impressions of the French Alps Given Moffat Three Days in Buttermerc C. L. Corbett Return to the Alps Freda Rylatt 70 Snowdonia My Measuring Stick E. Worsley Leech 74 Alpine Scotland E. M. Gregory 78 Autumn Days in Derbyshire Nancy Carpenter 82 Idle Thoughts of an Idle Climber Phy His Raven 86 Excursion into Ethnology H. C. Bryan 91 Club Notes A. Wilson 94 Obituary ; Reviews ; Editor's Note 95 to 102 Copies may be obtained from : Hon. Editor: Mrs. H. C BRYAN, Rosebank, Blakemere, Hereford. Hon. Secretary: Miss M. WOOD, i Grove Avenue, Frizinghall, Bradford. PUBLISHED BY THE PINNACLE CLUB © Pinnacle Club and Author All Rights Reserved THE PINNACLE CLUB JOURNAL 1950 © Pinnacle Club and Author All Rights Reserved ' —. r: H a © Pinnacle Club and Author All Rights Reserved The Pinnacle Club Journal 1950 EDITED BY HELEN BRYAN STOCKPORT THE CLOISTER PRESS LIMITED © Pinnacle Club and Author All Rights Reserved r.vn.vx LOWF. (\[RS. WORSI.FY LEFCH) PRESIDENT 1946-^9 © Pinnacle Club and Author All Rights Reserved THE PINNACLE CLUB 1950 OFFICERS AND COMMITTEE : President : Miss E. WELLS Westmead, Moorland View, Ben Rhydding, Yorks. Vice-President: Mrs. E. -

Environmentally Significant Areas Inventory of The
Environmentally Significant Areas Inventory of the Rocky Mountain Natural Region of Alberta Final Report by Kevin Timoney Treeline Ecological Research 21551 Twp. Rd. 520 Sherwood Park, AB T8E 1E3 email: [email protected] for Corporate Management Service Alberta Environmental Protection 12th Floor, Oxbridge Place 9820 - 106 St. Edmonton, AB T5K 2J6 17 January 1998 Contents ___________________________________________________________________ Abstract........................................................................................................................................ 1 Acknowledgements................................................................................................................... 2 Color Plates................................................................................................................................. 3 1. Purpose of the study ........................................................................................................... 6 1.1 Definition of AESA@................................................................................................... 6 1.2 Study Rationale ............................................................................................................ 6 2. Background on the Rocky Mountain Natural Region ............................................ 7 2.1 Geology ......................................................................................................................... 7 2.2 Weather and Climate................................................................................................... -

Squandering Paradise?
THREATS TO PROTECTED AREAS SQUANDERING PARADISE? The importance and vulnerability of the world’s protected areas By Christine Carey, Nigel Dudley and Sue Stolton Published May 2000 By WWF-World Wide Fund For Nature (Formerly World Wildlife Fund) International, Gland, Switzerland Any reproduction in full or in part of this publication must mention the title and credit the above- mentioned publisher as the copyright owner. © 2000, WWF - World Wide Fund For Nature (Formerly World Wildlife Fund) ® WWF Registered Trademark WWF's mission is to stop the degradation of the planet's natural environment and to build a future in which humans live in harmony with nature, by: · conserving the world's biological diversity · ensuring that the use of renewable natural resources is sustainable · promoting the reduction of pollution and wasteful consumption Front cover photograph © Edward Parker, UK The photograph is of fire damage to a forest in the National Park near Andapa in Madagascar Cover design Helen Miller, HMD, UK 1 THREATS TO PROTECTED AREAS Preface It would seem to be stating the obvious to say that protected areas are supposed to protect. When we hear about the establishment of a new national park or nature reserve we conservationists breathe a sigh of relief and assume that the biological and cultural values of another area are now secured. Unfortunately, this is not necessarily true. Protected areas that appear in government statistics and on maps are not always put in place on the ground. Many of those that do exist face a disheartening array of threats, ranging from the immediate impacts of poaching or illegal logging to subtle effects of air pollution or climate change. -

HCVF-V3-October 29-2014-Final
HIGH CONSERVATION VALUE FOREST ASSESSMENT Spray Lake Sawmills (1980) Ltd. Forest Management Agreement and B9 Quota Area Prepared for: Spray Lake Sawmills (1980) Ltd. Cochrane, Alberta Prepared by: John L. Kansas, M.Sc., P.Biol. HAB-TECH Environmental Ltd. Jason Mogilefsky, B.Sc., RPF Spray Lake Sawmills October 29, 2014 EXECUTIVE SUMMARY This report presents results of an assessment for the presence of High Conservation Value (HCV) attributes and forests on the Spray Lake Sawmills (SLS) Forest Management Agreement area (FMA) and B9 Quota land tenure. The assessment is a component of Principle 9, required for Forest Stewardship Council (FSC®) forest management certification. The High Conservation Value Forest (HCVF) concept focuses on environmental, social, and cultural values that make a particular forest area outstandingly significant. The assessment framework is organized as a table with 6 categories, which form the definition of a HCVF. Each Category includes a series of Key, Definitive and Guidance questions designed to help identify HCVF values and thresholds for HCVF designation. Identification of the high conservation values facilitates management decisions that are consistent with maintaining or enhancing the values. A HCVF possesses one or more of the following attributes: Category 1: Forest areas containing globally, regionally or nationally significant concentrations of biodiversity values (e.g., endemism, endangered species, refugia); Category 2: Forest areas containing globally, regionally or nationally significant large -

Castle Initial Project Description
Initial Project Description: Castle Project Teck Coal Limited Fording River Operations March 2020 Initial Project Description: Castle Project Executive Summary Introduction This document is an Initial Project Description (IPD) for the Teck Coal Limited (Teck) Fording River Operations Castle Project (the Castle Project or the Project) under the British Columbia (BC) Environmental Assessment Act (BC EAA) (SBC 2018, c 51). Together, the IPD and Engagement Plan (Appendix A) are used to initiate the Early Engagement Phase of the BC environmental assessment process. The purpose of the IPD is to provide information for interested parties to understand the Project and provide input to Teck. This allows feedback to be used to help shape the Project. The Engagement Plan includes a summary of all engagement conducted to date and outlines future engagement during the Early Engagement phase. Feedback on the IPD and Engagement Plan will be used to support the development of a Detailed Project Description (DPD). The DPD will in turn inform the Environmental Assessment Readiness Decision, while providing a degree of Project certainty and additional details from the IPD about project design to inform the Process Planning stage. The Process Planning stage sets the scope, methods and information requirements for the assessment and defines subsequent engagement approaches with interested parties. Fording River Operations (FRO) is a steelmaking coal mine in the Elk Valley in the East Kootenay Region of southeast BC. Beginning in the mid-2020s, less economically mineable coal will be available from the existing operating areas at FRO. Castle Mountain, located immediately south of the current mining operations at FRO, has extensive deposits of mineable steelmaking coal and represents a logical extension of FRO. -

The Grasslands of British Columbia
The Grasslands of British Columbia The Grasslands of British Columbia Brian Wikeem Sandra Wikeem April 2004 COVER PHOTO Brian Wikeem, Solterra Resources Inc. GRAPHICS, MAPS, FIGURES Donna Falat, formerly Grasslands Conservation Council of B.C., Kamloops, B.C. Ryan Holmes, Grasslands Conservation Council of B.C., Kamloops, B.C. Glenda Mathew, Left Bank Design, Kamloops, B.C. PHOTOS Personal Photos: A. Batke, Andy Bezener, Don Blumenauer, Bruno Delesalle, Craig Delong, Bob Drinkwater, Wayne Erickson, Marylin Fuchs, Perry Grilz, Jared Hobbs, Ryan Holmes, Kristi Iverson, C. Junck, Bob Lincoln, Bob Needham, Paul Sandborn, Jim White, Brian Wikeem. Institutional Photos: Agriculture Agri-Food Canada, BC Archives, BC Ministry of Forests, BC Ministry of Water, Land and Air Protection, and BC Parks. All photographs are the property of the original contributor and can not be reproduced without prior written permission of the owner. All photographs by J. Hobbs are © Jared Hobbs. © Grasslands Conservation Council of British Columbia 954A Laval Crescent Kamloops, B.C. V2C 5P5 http://www.bcgrasslands.org/ All rights reserved. No part of this document or publication may be reproduced in any form without prior written permission of the Grasslands Conservation Council of British Columbia. ii Dedication This book is dedicated to the Dr. Vernon pathfinders of our ecological Brink knowledge and understanding of Dr. Alastair grassland ecosystems in British McLean Columbia. Their vision looked Dr. Edward beyond the dust, cheatgrass and Tisdale grasshoppers, and set the course to Dr. Albert van restoring the biodiversity and beauty Ryswyk of our grasslands to pristine times. Their research, extension and teaching provided the foundation for scientific management of our grasslands. -
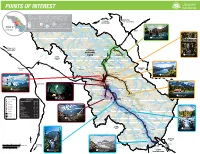
Points of Interest
POINTS OF INTEREST With over 11 000 km2 of wild and lush terrain, it’s safe to say Jasper National Park is rather large. The best Edson way to explore this incredible landscape is to take a day Grande Cache Edmonton ROCK LAKE Grande Prairie and explore each of its 5 major regions: SOLOMON CREEK Scenic road to Alaska 16 WILDLAND PARK 1 Around Town, 2 Maligne Valley, 3 Edith Cavell and 93A, 40 4 Miette Hot Springs and 5 the Icefields Parkway. Each of TOP 5 these regions contain very unique terrain, water ways, wilderness REGIONS and facilities. Hinton Snake Indian River Miette Hot Springs 61 km from Jasper Moosehorn Creek Snake Indian River 40 47 ALBERTA BRITISH COLUMBIA Roche Ronde Prince George Fort St. James Athabasca River Prince Rupert JASPER Pocahontas Syncline RidgeRoche Miette Fiddle River NATIONAL Miette 2 Maligne Canyon 16 PARK Hot Springs Mount Robson MOUNT 40 11.5 km from Jasper Mount Greenock Sulphur Skyline ROBSON Talbot PROVINCIAL Jasper Lake Lake Utopia Mountain PARK Esplanade Mountain Snaring River 16 40 Cinquefoil Mountain Rocky River Morro Peak Overlander Falls Hawk Mountain Snaring Mount Colin 16 WHITEHORSE Tête Jaune Rearguard WILDERNESS Falls Cache Mount Bridgland AREA Victoria Cross Ranges Pyramid Mountain Roche Bonhomme Pyramid 5 Maligne Canyon 2 Lake Medicine Lake Colin Range Maligne River 27 km from Jasper Miette River Jasper Yellowhead Lake 16 Old Fort Point Signal Mountain Wapiti Mount Tekarra Medicine Lake Valemount Whistlers Queen Elizabeth Range Marmot 93 Athabasca River Curator Mountain 1 Around Town See back of map Wabasso Opal Peak Tonquin Mount Clitheroe Maligne Valley Mount Hardisty Lake 2 Mount Maccarib Maligne Lake Bald Hills The Ramparts Edith 93A 48 km from Jasper Legend 3 Cavell Highway 93A Athabasca Maligne River Astoria River Mount Maligne Information Centre 7 km from Jasper Falls Lake DISTANCES Edith Cavell Mount Kerkeslin Camping Jasper Townsite to.. -

ICEFIELDS PARKWAY Driving Guide
ICEFIELDS PARKWAY driving guide an eBook from Parkways of the Canadian Rockies, the region’s original and most comprehensive driving guide | by Brian Patton CONTENTS 2 Herbert Lake .................................................................................................................. 4 Crowfoot Glacier ....................................................................................................... 6 Bow Lake ........................................................................................................................... 7 Peyto Lake ........................................................................................................................ 9 Upper Waterfowl Lake ........................................................................................ 11 Lower Waterfowl Lake ........................................................................................ 11 Mistaya Canyon ........................................................................................................... 12 David Thompson Highway ............................................................................... 14 Rampart Creek ............................................................................................................. 15 Graveyard Flats ............................................................................................................ 15 Weeping Wall ............................................................................................................... 16 Big Bend ............................................................................................................................ -

Lnf Ormal'lon to USERS
lNfORMAl'lON TO USERS This manusdpt has been mpmduœd fm the m~lmmaster. UMI films the text diredly from the original or mpy submiüed. Thus, rome thesis and dissertation wpies are in typewriter face, Mile others may be from any type of cornputer pnnter. The qurlity of thb rsprodudkn k d.p.nd.nt upon the qurlity d ai. copy subrnilted. Broken or indistaid mnt, coîored or poor quality ükistrati6ns and photogrâphs, print Meedthrwgh, subsfandard margins, and improper alignment can adversely affect re-. ln the unlikety event Ihat the author diid not send UMI a complebe muscript and thete are missing pages, them will b8 noted, Also, if unauthorired copyright material had to be mmoved, a note wifl indïïe the deletlon. Oversize materials (8.0.. maps, drawings, charts) am rsproduced by sectiming the original, beginning at üw upper lefthand corner ai'@continuing from ieft to right in equal sedons mtn srnail overtaps. Photographs included in the original manusui@ have been reqmâuœâ xemgraphically in this copy. HigMquality 6. x W bîack and white photognphic prints are avaibbk for any phatographs or illusnatioris -ring in this co~yfor an additional charge. Cmtad UMI diredo order. Bell & Hawell Information and Leaming 300 North Zeeb Road, Ann Arbor, MI 48106-1348 USA Depositional environments, provenance and sequence stratigraphy of the type Sassenach Formation, Jasper, Alberta. - Sherry Becker Department of Earth and Planetary Sciences McGill University, Montreal July 1997 A thesis submitted to the Faculty of Graduate Studies and Research in partial fulfilment of the requirements of the degree of Masters of Science. 8 Sherry Becker 1997 National Library BiMiothèque nationale 1+1 of cana, du Canada Acquisitions and Acquisitions et Bibliographie Services sentices bibliographiques 395 Wellington Street 395. -

Maintenance Rebuild of the Altalink 551L Transmission Line Between Banff and Lake Louise
Maintenance rebuild of the AltaLink 551L transmission line between Banff and Lake Louise: Detailed Environmental Impact Analysis DRAFT Submitted by: Avens Consulting Inc. Canmore AB Submitted to: AltaLink LP Calgary AB And Parks Canada October 2015 AltaLink draft 551L Maintenance Rebuild EA AVENS CONSULTING In association with: Bruce Gleig – GIS Consultant Andrea Ram – GIS and Aquatics Report Authorship Project Manager: Rhonda DeLong, M.Sc., Avens Consulting. Wildlife: Mark Piorecky, M.Sc., Valhalla Environmental Consulting Inc. Vegetation: Rhonda DeLong, M.Sc., Avens Consulting Landforms and Soil: Matt Johnson, C. Tech., Corvidae Environmental Consulting Inc. Aquatic resources and hydrology: Andrea Ram, M.GIS, Ram Consulting Cultural resources: Neil Mirau and Don Boras, Arrow Archaeology Visibility analysis and GIS mapping: Bruce Gleig, B.E.S, GIS Consultant Avens Consulting September 2015 i AltaLink draft 551L Maintenance Rebuild EA Table of Contents 1. Project ..................................................................................................................... 1 1.1. Background ........................................................................................................................................1 1.2. Purpose and Justification .................................................................................................................2 1.3. Scope of Project .................................................................................................................................4 1.4. Scope