Life-Cycle Analysis Update of Glass and Glass Fiber for the GREETTM Model
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aremco—High Temperature Solutions
High Temperature Solutions Since 1965, our success has been a result of this simple business strategy: • Understanding Customer Requirements. • Providing Outstanding Service and Support. • Producing High Quality Technical Materials and Equipment. • Solving Difficult Technical Problems. CONTENTS Technical Bulletin Page No. A1 Machinable & Dense Ceramics .......................................................... 2 A2 High Temperature Ceramic & Graphite Adhesives ....................... 6 A3 High Temperature Ceramic-Metallic Pastes .................................12 A4 High Temperature Potting & Casting Materials ...........................14 A5-S1 High Temperature Electrical Coatings & Sealants ......................18 A5-S2 High Temperature High Emissivity Coatings ................................20 A5-S3 High Temperature Thermal Spray Sealants ..................................22 A5-S4 High Temperature Coatings for Ceramics, Glass & Quartz ......24 A5-S5 High Temperature Refractory Coatings .........................................26 A6 High Temperature Protective Coatings ..........................................28 A7 High Performance Epoxies ................................................................34 A8 Electrically & Thermally Conductive Materials .............................36 A9 Mounting Adhesives & Accessories ................................................38 A10 High Temperature Tapes ...................................................................42 A11 High Temperature Inorganic Binders..............................................44 -

Fiber Optic Cable for VOICE and DATA TRANSMISSION Delivering Solutions Fiber Optic THAT KEEP YOU CONNECTED Cable Products QUALITY
Fiber Optic Cable FOR VOICE AND DATA TRANSMISSION Delivering Solutions Fiber Optic THAT KEEP YOU CONNECTED Cable Products QUALITY General Cable is committed to developing, producing, This catalog contains in-depth and marketing products that exceed performance, information on the General Cable quality, value and safety requirements of our line of fiber optic cable for voice, customers. General Cable’s goal and objectives video and data transmission. reflect this commitment, whether it’s through our focus on customer service, continuous improvement The product and technical and manufacturing excellence demonstrated by our sections feature the latest TL9000-registered business management system, information on fiber optic cable the independent third-party certification of our products, from applications and products, or the development of new and innovative construction to detailed technical products. Our aim is to deliver superior performance from all of General Cable’s processes and to strive for and specific data. world-class quality throughout our operations. Our products are readily available through our network of authorized stocking distributors and distribution centers. ® We are dedicated to customer TIA 568 C.3 service and satisfaction – so call our team of professionally trained sales personnel to meet your application needs. Fiber Optic Cable for the 21st Century CUSTOMER SERVICE All information in this catalog is presented solely as a guide to product selection and is believed to be reliable. All printing errors are subject to General Cable is dedicated to customer service correction in subsequent releases of this catalog. and satisfaction. Call our team of professionally Although General Cable has taken precautions to ensure the accuracy of the product specifications trained sales associates at at the time of publication, the specifications of all products contained herein are subject to change without notice. -

Fluidized Bed Chemical Vapor Deposition of Zirconium Nitride Films
INL/JOU-17-42260-Revision-0 Fluidized Bed Chemical Vapor Deposition of Zirconium Nitride Films Dennis D. Keiser, Jr, Delia Perez-Nunez, Sean M. McDeavitt, Marie Y. Arrieta July 2017 The INL is a U.S. Department of Energy National Laboratory operated by Battelle Energy Alliance INL/JOU-17-42260-Revision-0 Fluidized Bed Chemical Vapor Deposition of Zirconium Nitride Films Dennis D. Keiser, Jr, Delia Perez-Nunez, Sean M. McDeavitt, Marie Y. Arrieta July 2017 Idaho National Laboratory Idaho Falls, Idaho 83415 http://www.inl.gov Prepared for the U.S. Department of Energy Under DOE Idaho Operations Office Contract DE-AC07-05ID14517 Fluidized Bed Chemical Vapor Deposition of Zirconium Nitride Films a b c c Marie Y. Arrieta, Dennis D. Keiser Jr., Delia Perez-Nunez, * and Sean M. McDeavitt a Sandia National Laboratories, Albuquerque, New Mexico 87185 b Idaho National Laboratory, Idaho Falls, Idaho 83401 c Texas A&M University, Department of Nuclear Engineering, College Station, Texas 77840 Received November 11, 2016 Accepted for Publication May 23, 2017 Abstract — – A fluidized bed chemical vapor deposition (FB-CVD) process was designed and established in a two-part experiment to produce zirconium nitride barrier coatings on uranium-molybdenum particles for a reduced enrichment dispersion fuel concept. A hot-wall, inverted fluidized bed reaction vessel was developed for this process, and coatings were produced from thermal decomposition of the metallo-organic precursor tetrakis(dimethylamino)zirconium (TDMAZ) in high- purity argon gas. Experiments were executed at atmospheric pressure and low substrate temperatures (i.e., 500 to 550 K). Deposited coatings were characterized using scanning electron microscopy, energy dispersive spectroscopy, and wavelength dis-persive spectroscopy. -

Acrylic Polymer Transparencies
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 4-1972 Acrylic Polymer Transparencies Inez Allen Kendrick Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Art Education Commons, Art Practice Commons, and the Interdisciplinary Arts and Media Commons Let us know how access to this document benefits ou.y Recommended Citation Kendrick, Inez Allen, "Acrylic Polymer Transparencies" (1972). Dissertations and Theses. Paper 1554. https://doi.org/10.15760/etd.1553 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. AN ABSTRACT OF T.HE THESIS OF Inez Allen Kendrick tor the Master ot Science in Teaching in Art presented April 26, 1972. TITLE: Acrylic Polymer Transparencies. APPROVED BY MEMBERS 01' 'lBE THESIS COMMITTEE: Richard J. ~asch, Chairman ~d B. Kimbrell Robert S. Morton Brief mentions by three writers on synthetic painting media first intrigued my interest in a' new technique of making transparent acrylic paintings on glass or plexiglas supports, some ot which were said to I I simulate stained-glass windows. In writing this paper on acrylic polymer'", transparencies, 'my problem was three-told: first. to determine whether any major recognized works of art have been produced by this, method; second, to experiment with the techni'que and materials in order to explore their possibilities for my own work; and third, to determine whether both materials and methods would be suitable for use in a classroom. -

Thermal Runaway Severity Reduction Assessment for EVA Li-Ion Batteries
https://ntrs.nasa.gov/search.jsp?R=20150018568 2019-08-31T06:01:42+00:00Z Thermal Runaway Severity Reduction Assessment For EVA Li-ion Batteries By Eric Darcy/NASA-JSC For 2014 JSC Connect Event Energy Storage & Management 24 Sept 2014 Team and Contents 2 • NASA Engineering Safety Center Lead Effort – Paul Shack, Assessment Lead – Chris Iannello, NESC Technical Fellow for Electrical Power – Steve Rickman, NESC Technical Fellow for Passive Thermal – Eric Darcy, Test Lead for EVA Batteries, NASA-JSC – Sam Russell, Mike Fowler, Judy Jeevarajan, Craig Clark, John Weintritt, Christina Deoja and Stacie Cox/NASA-JSC – Rob Button, Tom Miller, Penni Dalton/NASA-GRC – Dan Doughty, Bruce Drolen, Ralph White, Gary Bayles, and Jim Womack/NESC Consultants • Agenda – Background on the EVA batteries – Motivation and objectives – Trigger method selected and why – Assessments of current designs – Verification of subscale mitigation measures – Full scale LREBA with those measures leads to failure – Consequence of cell TR ejecta products to TR propagation – Full scale LREBA with adjacent cells protected from cell vent path – Bank test to verify benefits of cell fusing – Lessons learned to date Background - Li-ion Rechargeable EVA Battery Assembly (LREBA) 1 9P-5S Array of Samsung 2.6Ah 18650 cells to power the spacesuit helmet lights and camera and glove heaters Background – Li-ion Pistol Grip Tool Battery • 10-cell Li-ion 18650 battery – 10S for discharge – 2P-5S for charge • Battery is enclosed in tool holster except for end with the D-latch Background -
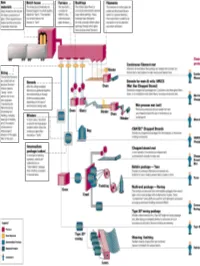
All About Fibers
RawRaw MaterialsMaterials ¾ More than half the mix is silica sand, the basic building block of any glass. ¾ Other ingredients are borates and trace amounts of specialty chemicals. Return © 2003, P. Joyce BatchBatch HouseHouse && FurnaceFurnace ¾ The materials are blended together in a bulk quantity, called the "batch." ¾ The blended mix is then fed into the furnace or "tank." ¾ The temperature is so high that the sand and other ingredients dissolve into molten glass. Return © 2003, P. Joyce BushingsBushings ¾The molten glass flows to numerous high heat-resistant platinum trays which have thousands of small, precisely drilled tubular openings, called "bushings." Return © 2003, P. Joyce FilamentsFilaments ¾This thin stream of molten glass is pulled and attenuated (drawn down) to a precise diameter, then quenched or cooled by air and water to fix this diameter and create a filament. Return © 2003, P. Joyce SizingSizing ¾The hair-like filaments are coated with an aqueous chemical mixture called a "sizing," which serves two main purposes: 1) protecting the filaments from each other during processing and handling, and 2) ensuring good adhesion of the glass fiber to the resin. Return © 2003, P. Joyce WindersWinders ¾ In most cases, the strand is wound onto high-speed winders which collect the continuous fiber glass into balls or "doffs.“ Single end roving ¾ Most of these packages are shipped directly to customers for such processes as pultrusion and filament winding. ¾ Doffs are heated in an oven to dry the chemical sizing. Return © 2003, P. Joyce IntermediateIntermediate PackagePackage ¾ In one type of winding operation, strands are collected into an "intermediate" package that is further processed in one of several ways. -

Stability of Materials for Use in Space-Based Interferometric Missions
STABILITY OF MATERIALS FOR USE IN SPACE-BASED INTERFEROMETRIC MISSIONS By ALIX PRESTON A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2010 1 °c 2010 Alix Preston 2 This is dedicated to all who were told they would fail, only to prove them wrong 3 ACKNOWLEDGMENTS Much of this work would not have been made possible if it were not for the help of many graduate and undergraduate students, faculty, and sta®. I would like to thank Ira Thorpe, Rachel Cruz, Vinzenz Vand, and Josep Sanjuan for their help and thoughtful discussions that were instrumental in understanding the nuances of my research. I would also like to thank Gabriel Boothe, Aaron Spector, Benjamin Balaban, Darsa Donelon, Kendall Ackley, and Scott Rager for their dedication and persistence to getting the job done. A special thanks is due for the physics machine shop, especially Marc Link and Bill Malphurs, who spent many hours on the countless projects I needed. Lastly, I would like to thank my advisor, Dr. Guido Mueller, who put up with me, guided me, and supported me in my research. 4 TABLE OF CONTENTS page ACKNOWLEDGMENTS ................................. 4 LIST OF TABLES ..................................... 9 LIST OF FIGURES .................................... 10 KEY TO ABBREVIATIONS ............................... 17 KEY TO SYMBOLS .................................... 19 ABSTRACT ........................................ 20 CHAPTER 1 INTRODUCTION .................................. 22 1.1 Space-Based Missions .............................. 23 1.2 GRACE ..................................... 23 1.3 GRACE Follow-On ............................... 25 1.4 LISA ....................................... 26 1.4.1 Introduction ............................... 26 1.4.2 Sources .................................. 27 1.4.2.1 Cosmological background sources ............. -

The Materials Science and Engineering of Advanced YB-Doped Glasses and Fibers for High-Power Lasers
Clemson University TigerPrints All Dissertations Dissertations May 2020 The Materials Science and Engineering of Advanced YB-Doped Glasses and Fibers for High-Power Lasers Thomas Wade Hawkins Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Recommended Citation Hawkins, Thomas Wade, "The Materials Science and Engineering of Advanced YB-Doped Glasses and Fibers for High-Power Lasers" (2020). All Dissertations. 2585. https://tigerprints.clemson.edu/all_dissertations/2585 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. THE MATERIALS SCIENCE AND ENGINEERING OF ADVANCED YB-DOPED GLASSES AND FIBERS FOR HIGH-POWER LASERS A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Materials Science and Engineering by Thomas Wade Hawkins May 2020 Accepted by: Dr. Liang Dong, Committee Chair Dr. John Ballato Dr. Peter Dragic Dr. Stephen Foulger Dr. Philip Brown ABSTRACT This research studies and yields new understandings into the materials science and engineering of advanced multicomponent glass systems, which is critical for next generation fiber lasers operating at high output powers. This begins with the study and development of Yb-doped glasses in the Al2O3-P2O5-SiO2 (APS) ternary system, fabricated using modified chemical vapor deposition (MCVD), that, despite being highly doped, possess an average refractive index matched to that of silica (SiO2). The highly doped active core material was subsequently processed through a multiple stack-and- draw process to realize a single fiber with high doping, compositionally-tailored index, and scalability for fiber lasers. -

Femtosecond Laser Direct-Write of Optofluidic Lab in Fiber Through Polymer-Coated Optical Fibers
Femtosecond Laser Direct-Write of Optofluidic Lab in Fiber through Polymer-Coated Optical Fibers by Kevin A. J. Joseph A thesis submitted in conformity with the requirements for the degree of Master of Applied Science Graduate Department of The Edward S. Rogers Sr. Department of Electrical and Computer Engineering University of Toronto Copyright c 2017 by Kevin A. J. Joseph Abstract Femtosecond Laser Direct-Write of Optofluidic Lab in Fiber through Polymer-Coated Optical Fibers Kevin A. J. Joseph Master of Applied Science Graduate Department of The Edward S. Rogers Sr. Department of Electrical and Computer Engineering University of Toronto 2017 Three-dimensional femtosecond laser processing of lab in fiber, the combination of integrated photonics and microfluidics inside optical fiber, was demonstrated in silica glass fibers coated with polymer buffer. This enables the laser fabrication of lab-on- chip functionalities onto optical fiber without the time-consuming and mechanically- compromising process of buffer removal. In this thesis, an assessment of laser-induced damage in buffer-coated fiber is reported, along with methods to avoid and mitigate this damage. Further, selective buffer machining is studied enabling in-tandem processing of the glass fiber core and cladding with the polymer buffer. Structuring of the fiber core, cladding photonic circuits, and microfluidics were demonstrated in buffer-coated fiber without removal of or damage to the polymer buffer. The methods and processes here make the lab in fiber platform to be more industry viable and opens new opportunities for device architectures spanning across the fiber core, cladding, and buffer, representing a significant technological advancement. -

Fiber Reinforcement of a Resin Modified Glass Ionomer Cement Abstract Objectives. Understand How Discontinuous Short Glass Fiber
Fiber reinforcement of a resin modified glass ionomer cement a b b a Carina B. Tanaka , Frances Ershad , Ayman Ellakwa , Jamie J. Kruzic * a - School of Mechanical and Manufacturing Engineering, University of New South Wales (UNSW Sydney), Sydney NSW 2052, Australia b - School of Dentistry, The University of Sydney, Westmead NSW 2145, Australia *Corresponding author. Jamie J. Kruzic Address: School of Mechanical and Manufacturing Engineering, University of New South Wales (UNSW Sydney), Sydney NSW 2052, Australia. Tel.: +61 2 9385 4017 E-mail address: [email protected] Abstract Objectives. Understand how discontinuous short glass fibers and braided long fibers can be effectively used to reinforce a resin modified glass ionomer cement (RMGIC) for carious lesions restorations. Methods. Two control groups (powder/liquid kit and capsule) were prepared from a light cured RMGIC. Either discontinuous short glass fibers or braided polyethylene fiber ribbons were used as a reinforcement both with and without pre-impregnation with resin. For the former case, the matrix was the powder/liquid kit RMGIC, and for the latter case the matrix was the capsule form. Flexural strength was evaluated by three-point beam bending and fracture toughness was evaluated by the single-edge V-notch beam method. Compressive strength tests were performed on cylindrical samples. Results were compared by analysis of variances and Tukey’s post-hoc test. Flexural strength data were analyzed using Weibull statistical analysis. Results. The short fiber reinforced RMGIC both with and without pre-impregnation showed a significant increase of ~50% in the mean flexural strength and 160 – 220% higher fracture toughness compared with the powder/liquid RMGIC control. -

Chemical Vapor Deposition of Silanes and Patterning on Silicon
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2010-12-15 Chemical Vapor Deposition of Silanes and Patterning on Silicon Feng Zhang Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Biochemistry Commons, and the Chemistry Commons BYU ScholarsArchive Citation Zhang, Feng, "Chemical Vapor Deposition of Silanes and Patterning on Silicon" (2010). Theses and Dissertations. 2902. https://scholarsarchive.byu.edu/etd/2902 This Dissertation is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Chemical Vapor Deposition of Silanes and Patterning on Silicon Feng Zhang A dissertation submitted to the faculty of Brigham Young University In partial fulfillment of the requirements for the degree of Doctor of Philosophy Matthew R. Linford Milton L. Lee Matthew C. Asplund Daniel E. Austin Robert C. Davis Department of Chemistry and Biochemistry Brigham Young University April 2011 Copyright © 2011 Feng Zhang All Rights Reserved ABSTRACT Chemical Vapor Deposition of Silanes and Patterning on Silicon Feng Zhang Department of Chemistry and Biochemistry Doctor of Philosophy Self assembled monolayers (SAMs) are widely used for surface modification. Alkylsilane monolayers are one of the most widely deposited and studied SAMs. My work focuses on the preparation, patterning, and application of alkysilane monolayers. 3-aminopropyltriethoxysilane (APTES) is one of the most popular silanes used to make active surfaces for surface modification. To possibly improve the surface physical properties and increase options for processing this material, I prepared and studied a series of amino silane surfaces on silicon/silicon dioxide from APTES and two other related silanes by chemical vapor deposition (CVD). -

Process Model and Control System for the Glass Fiber Drawing Process
Graduate Theses, Dissertations, and Problem Reports 2010 Process Model and Control System for the Glass Fiber Drawing Process Songke Liu West Virginia University Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Liu, Songke, "Process Model and Control System for the Glass Fiber Drawing Process" (2010). Graduate Theses, Dissertations, and Problem Reports. 3006. https://researchrepository.wvu.edu/etd/3006 This Dissertation is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Dissertation has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. Process Model and Control System for the Glass Fiber Drawing Process Songke Liu Dissertation submitted to the College of Engineering and Mineral Resources at West Virginia University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Mechanical Engineering Larry E. Banta, Ph. D., Chair Wade W. Huebsch, Ph. D. Kenneth H. Means, Ph. D. Mario Perhinschi, Ph. D. Natalia A. Schmid, Ph. D. Department of Mechanical and Aerospace Engineering Morgantown, West Virginia 2010 Keywords: Glass Fiber; Drawing Process; Temperature Estimation; Process Control Copyright 2010 Songke Liu Abstract Process Model and Control System for the Glass Fiber Drawing Process Songke Liu Drawing of glass fibers is an important industrial process used for manufacture of a variety of materials ranging from optical communications cables to fiber filter media.