Molecular Mechanisms of Phytochrome Signal Transduction in Higher Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phytochrome Effects in the Nyctinastic Leaf Movements of Albizzia Julibrissin and Some Other Legumes1 2 William S
Plant Physiol. (1967) 42, 1413-1418 Phytochrome Effects in the Nyctinastic Leaf Movements of Albizzia julibrissin and Some Other Legumes1 2 William S. Hillman and Willard L. Koukkari Biology Department, Brookhaven National Laboratory, Upton, New York 11973 Received June 5, 1967. Summnary. Participation of phytochrome 'is evident in the nyctinastic responise of leaves of Albizzia julibrissin (silk-tree), Albizzia lophantha, Leucaena glauca, Poinciana gilliesi and Calliandra inequilatera; closure of excised pairs of pinnules upon darkening is rapid following red illumination and slow following far-red. Under good conditions the difiference is obvious within 10 minutes. These observations conifirm a report by Fondeville, Borthwick, and Hendricks on the sensitive plant, Mimosa pudica, but indicate that the efifect bears no necessary relationship to the anomalous sensitivity of Mimosa. In A. julibrissin, phytochrome control is mnarked in experiments conducted early in the daily 12-hour light period and appears absent, or nearly so, toward the end of the light period, perhaps due to interaction with an endogenous circadian rhythm. Effects of leaf maturity and of the position of a pinnule-pair within a leaf are also evident. Tih-ese results are not easily reconciled with hypotheses of phytochrome action through gene activation and nucleic acid synthesis, but are consistent with hypothess ibased onl permeability changes and membrane properties. The mgnitude and reproducibility of the response in A. jutlibrissin suggest its use as a lajboratory exercise; this and related systems should prove valuable for eventuai identification of the mechanism of phytochrome action. Fondeville, Borthwick, and Hendricks (2) re- pinnately twice-compound leaves generally similar in ported on a role of phytochrome in the nyctinastic character to those of Mimosa pudica, (but not obviously response of the sensitive plant, Mimnosa pudica: closure sensitive to the touch. -

Nobel Lecture by Roger Y. Tsien
CONSTRUCTING AND EXPLOITING THE FLUORESCENT PROTEIN PAINTBOX Nobel Lecture, December 8, 2008 by Roger Y. Tsien Howard Hughes Medical Institute, University of California San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0647, USA. MOTIVATION My first exposure to visibly fluorescent proteins (FPs) was near the end of my time as a faculty member at the University of California, Berkeley. Prof. Alexander Glazer, a friend and colleague there, was the world’s expert on phycobiliproteins, the brilliantly colored and intensely fluorescent proteins that serve as light-harvesting antennae for the photosynthetic apparatus of blue-green algae or cyanobacteria. One day, probably around 1987–88, Glazer told me that his lab had cloned the gene for one of the phycobilipro- teins. Furthermore, he said, the apoprotein produced from this gene became fluorescent when mixed with its chromophore, a small molecule cofactor that could be extracted from dried cyanobacteria under conditions that cleaved its bond to the phycobiliprotein. I remember becoming very excited about the prospect that an arbitrary protein could be fluorescently tagged in situ by genetically fusing it to the phycobiliprotein, then administering the chromophore, which I hoped would be able to cross membranes and get inside cells. Unfortunately, Glazer’s lab then found out that the spontane- ous reaction between the apoprotein and the chromophore produced the “wrong” product, whose fluorescence was red-shifted and five-fold lower than that of the native phycobiliprotein1–3. An enzyme from the cyanobacteria was required to insert the chromophore correctly into the apoprotein. This en- zyme was a heterodimer of two gene products, so at least three cyanobacterial genes would have to be introduced into any other organism, not counting any gene products needed to synthesize the chromophore4. -

Phytochrome and Photosystem I Interaction in a High-Energy
Proc. Nat. Acad. Sci. USA Vol. 69, No. 8, pp. 2150-2154, August 1972 Phytochrome and Photosystem I Interaction in a High-Energy Photoresponse (photosynthesis/photomorphogenesis/anthocyanin/turnip seedlings) MICHAEL SCHNEIDER AND WILLIAM STIMSON Department of Biological Sciences, Columbia University, New York, N.Y. 10027 Communicated by Sterling B. Hendricks, May £6, 1972 ABSTRACT At least two photoreactions can be demon- HER are based upon phytochrome as the sole strated in plant developmental responses: the low-energy photoreceptor. requiring phytochrome system and the high energy reac- Accordingly, HER are believed to arise through the mainte- tion. The action of these photoreactions on the formation nance of a low level of Pfr over a prolonged time. Indeed, the of anthocyanin by turnip seedlings is discussed. The syn- phytochrome and HER photoreactions appear closely linked, thesis of small amounts of anthocyanin can be controlled since photoresponses that exhibit evidence of a HER also solely by phytochrome, as evidenced by the red-far-red exhibit photoreversible effect of brief irradiations. Appreciable phytochrome photoreversibility under appropriate synthesis requires prolonged irradiations, the duration of conditions. The dependence of HER photoresponses on in- irradiation being more important than intensity. The tensity remains more difficult to explain, and is the principal data presented suggest that the energy dependence of subject of this communication. anthocyanin synthesis arises through photosynthesis. A Originally, the involvement of photosynthesis in HER mechanism for the interaction between photosynthesis was and phytochrome is suggested. Under conditions of natural responses suggested by Hendricks, Borthwick, and their illumination of plants, the concentration of the species of associates (5, 13, 14). -

Phytochrome-Mediated Photoperception and Signal Transduction in Higher Plants
EMBO reports Phytochrome-mediated photoperception and signal transduction in higher plants Eberhard Schäfer & Chris Bowler1,+ Universitat Freiburg, Institut fur Biologie II/Botanik, Schanzlestrasse 1, D-79104 Freiburg, Germany and 1Molecular Plant Biology Laboratory, Stazione Zoologica ‘Anton Dohrn’, Villa Comunale, I-80121 Naples, Italy Received July 1, 2002; revised September 30, 2002; accepted October 1, 2002 Light provides a major source of information from the environ- Phytochromes are typically encoded by small multigene ment during plant growth and development. Light perception families, e.g. PHYA-PHYE in Arabidopsis (Møller et al., 2002; is mediated through the action of several photoreceptors, Nagy and Schäfer, 2002; Quail, 2002a,b). Each forms a including the phytochromes. Recent results demonstrate that homodimer of ∼240 kDa and light sensitivity is conferred by the light responses involve the regulation of several thousand presence of a tetrapyrrole chromophore covalently bound to the genes. Some of the key events controlling this gene expression N-terminal half of each monomer (Montgomery and Lagarias, are the translocation of the phytochrome photoreceptors into 2002). Dimerization domains are located within the C-terminal the nucleus followed by their binding to transcription factors. half of the proteins, as are other domains involved in the activa- Coupled with these events, the degradation of positively tion of signal transduction (Quail et al., 1995; Quail, 2002a). acting intermediates appears to be an important process Each phytochrome can exist in two photointerconvertible whereby photomorphogenesis is repressed in darkness. This conformations, denoted Pr (a red light-absorbing form) and Pfr review summarizes our current knowledge of these processes. (a far red light-absorbing form) (Figure 1A). -

Phytochrome Activates the Plastid-Encoded RNA Polymerase for Chloroplast Biogenesis Via Nucleus-To-Plastid Signaling
ARTICLE https://doi.org/10.1038/s41467-019-10518-0 OPEN Phytochrome activates the plastid-encoded RNA polymerase for chloroplast biogenesis via nucleus-to-plastid signaling Chan Yul Yoo 1, Elise K. Pasoreck1, He Wang1, Jun Cao2, Gregor M. Blaha3, Detlef Weigel2 & Meng Chen 1 Light initiates chloroplast biogenesis by activating photosynthesis-associated genes encoded by not only the nuclear but also the plastidial genome, but how photoreceptors control 1234567890():,; plastidial gene expression remains enigmatic. Here we show that the photoactivation of phytochromes triggers the expression of photosynthesis-associated plastid-encoded genes (PhAPGs) by stimulating the assembly of the bacterial-type plastidial RNA polymerase (PEP) into a 1000-kDa complex. Using forward genetic approaches, we identified REGULATOR OF CHLOROPLAST BIOGENESIS (RCB) as a dual-targeted nuclear/plastidial phytochrome sig- naling component required for PEP assembly. Surprisingly, RCB controls PhAPG expression primarily from the nucleus by interacting with phytochromes and promoting their localization to photobodies for the degradation of the transcriptional regulators PIF1 and PIF3. RCB- dependent PIF degradation in the nucleus signals the plastids for PEP assembly and PhAPG expression. Thus, our findings reveal the framework of a nucleus-to-plastid anterograde signaling pathway by which phytochrome signaling in the nucleus controls plastidial transcription. 1 Department of Botany and Plant Sciences, Institute for Integrative Genome Biology, University of California, -

Regulation of Phytochrome Gene Expression
Journal of the Iowa Academy of Science: JIAS Volume 98 Number Article 6 1991 Regulation of Phytochrome Gene Expression J. T. Colbert Colorado State University S. A. Costigan Colorado State University P. Avissar Colorado State University Z. Zhao Colorado State University Let us know how access to this document benefits ouy Copyright © Copyright 1991 by the Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/jias Part of the Anthropology Commons, Life Sciences Commons, Physical Sciences and Mathematics Commons, and the Science and Mathematics Education Commons Recommended Citation Colbert, J. T.; Costigan, S. A.; Avissar, P.; and Zhao, Z. (1991) "Regulation of Phytochrome Gene Expression," Journal of the Iowa Academy of Science: JIAS, 98(2), 63-67. Available at: https://scholarworks.uni.edu/jias/vol98/iss2/6 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Journal of the Iowa Academy of Science: JIAS by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. )our. Iowa Acad. Sci. 98(2):63-67, 1991 Regulation of Phytochrome Gene Expression J.T. COLBERT\ S.A. COSTIGAN2, P. AVISSAR3 and Z . ZHA04 Department of Biology, Colorado State University, Ft. Collins, CO 80523 In etiolated oat seedlings exposure to red light results in a decrease in the transcription of the phytochrome genes, the abundance of phytochrome mRNA, and the level of phytochrome protein. Phytochrome itself serves as the photoreceptor for the response of decreased mRNA and transcriprion levels. -

Sumoylation of Phytochrome-B Negatively Regulates Light-Induced Signaling in Arabidopsis Thaliana
SUMOylation of phytochrome-B negatively regulates light-induced signaling in Arabidopsis thaliana Ari Sadanandoma,1, Éva Ádámb, Beatriz Orosaa, András Vicziánb, Cornelia Klosec, Cunjin Zhanga, Eve-Marie Jossed, László Kozma-Bognárb, and Ferenc Nagyb,d,1 aSchool of Biological and Biomedical Sciences, University of Durham, Durham DH1 3LE, United Kingdom; bPlant Biology Institute, Biological Research Centre, H-6726 Szeged, Hungary; cInstitute of Botany, University of Freiburg, D-79104 Freiburg, Germany; and dInstitute of Molecular Plant Science, School of Biology, University of Edinburgh, Edinburgh EH9 3JR, United Kingdom Edited by George Coupland, Max Planck Institute for Plant Breeding Research, Cologne, Germany, and approved July 16, 2015 (received for review August 8, 2014) The red/far red light absorbing photoreceptor phytochrome-B (phyB) steps of phyB signaling include (i) inactivation or alteration of cycles between the biologically inactive (Pr, λmax, 660 nm) and active the substrate specificity of CONSTITUTIVE PHOTOMOR- (Pfr; λmax, 730 nm) forms and functions as a light quality and quantity PHOGENIC 1 (COP1) that targets proteins to degradation (10), controlled switch to regulate photomorphogenesis in Arabidopsis. (ii) degradation and/or modulation of the transcriptional activity At the molecular level, phyB interacts in a conformation-dependent of negative regulatory PIF TFs (11), and (iii) induction of trans- fashion with a battery of downstream regulatory proteins, including criptional cascades that modulate the expression of 2,500–3,000 PHYTOCHROME INTERACTING FACTOR transcription factors, and genes of the Arabidopsis genome (12). by modulating their activity/abundance, it alters expression pat- The number of phyB Pfr molecules quantitatively determines terns of genes underlying photomorphogenesis. -

Phytochrome Diversity in Green Plants and the Origin of Canonical Plant Phytochromes
ARTICLE Received 25 Feb 2015 | Accepted 19 Jun 2015 | Published 28 Jul 2015 DOI: 10.1038/ncomms8852 OPEN Phytochrome diversity in green plants and the origin of canonical plant phytochromes Fay-Wei Li1, Michael Melkonian2, Carl J. Rothfels3, Juan Carlos Villarreal4, Dennis W. Stevenson5, Sean W. Graham6, Gane Ka-Shu Wong7,8,9, Kathleen M. Pryer1 & Sarah Mathews10,w Phytochromes are red/far-red photoreceptors that play essential roles in diverse plant morphogenetic and physiological responses to light. Despite their functional significance, phytochrome diversity and evolution across photosynthetic eukaryotes remain poorly understood. Using newly available transcriptomic and genomic data we show that canonical plant phytochromes originated in a common ancestor of streptophytes (charophyte algae and land plants). Phytochromes in charophyte algae are structurally diverse, including canonical and non-canonical forms, whereas in land plants, phytochrome structure is highly conserved. Liverworts, hornworts and Selaginella apparently possess a single phytochrome, whereas independent gene duplications occurred within mosses, lycopods, ferns and seed plants, leading to diverse phytochrome families in these clades. Surprisingly, the phytochrome portions of algal and land plant neochromes, a chimera of phytochrome and phototropin, appear to share a common origin. Our results reveal novel phytochrome clades and establish the basis for understanding phytochrome functional evolution in land plants and their algal relatives. 1 Department of Biology, Duke University, Durham, North Carolina 27708, USA. 2 Botany Department, Cologne Biocenter, University of Cologne, 50674 Cologne, Germany. 3 University Herbarium and Department of Integrative Biology, University of California, Berkeley, California 94720, USA. 4 Royal Botanic Gardens Edinburgh, Edinburgh EH3 5LR, UK. 5 New York Botanical Garden, Bronx, New York 10458, USA. -

The Growing and Glowing Toolbox of Fluorescent and Photoactive Proteins
UC San Diego UC San Diego Previously Published Works Title The Growing and Glowing Toolbox of Fluorescent and Photoactive Proteins. Permalink https://escholarship.org/uc/item/6jx417t1 Journal Trends in biochemical sciences, 42(2) ISSN 0968-0004 Authors Rodriguez, Erik A Campbell, Robert E Lin, John Y et al. Publication Date 2017-02-01 DOI 10.1016/j.tibs.2016.09.010 Peer reviewed eScholarship.org Powered by the California Digital Library University of California HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Trends Biochem Manuscript Author Sci. Author Manuscript Author manuscript; available in PMC 2018 February 01. Published in final edited form as: Trends Biochem Sci. 2017 February ; 42(2): 111–129. doi:10.1016/j.tibs.2016.09.010. The growing and glowing toolbox of fluorescent and photoactive proteins Erik A. Rodriguez1, Robert E. Campbell2, John Y. Lin3, Michael Z. Lin4, Atsushi Miyawaki5, Amy E. Palmer6, Xiaokun Shu7, Jin Zhang1, and Roger Y. Tsien1,8 1Department of Pharmacology, University of California, San Diego, La Jolla, California, 92093, USA. 2Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2, Canada. 3School of Medicine, University of Tasmania, Hobart, Tasmania, 7000, Australia. 4Department of Bioengineering, Stanford University, Stanford, CA, 94305, USA and Department of Pediatrics, Stanford University, Stanford, CA, 94305, USA. 5Laboratory for Cell Function Dynamics, Brain Science Institute, RIKEN, 2-1 Hirosawa, Wako, Saitama, 351-0198, Japan. 6Department of Chemistry and Biochemistry, BioFrontiers Institute, University of Colorado Boulder, CO, 80303, USA. 7Department of Pharmaceutical Chemistry, University of California, San Francisco, San Francisco, CA, 94158, USA and Cardiovascular Research Institute, University of California, San Francisco, San Francisco, CA, 94158, USA. -
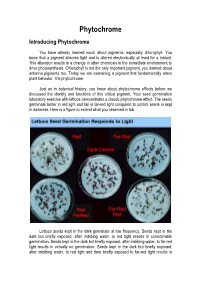
Phytochrome Introducing Phytochrome
Phytochrome Introducing Phytochrome You have already learned much about pigments, especially chlorophyll. You know that a pigment absorbs light and is altered electronically at least for a instant. This alteration results in a change in other chemicals in the immediate environment to drive photosynthesis. Chlorophyll is not the only important pigment, you learned about antenna pigments too. Today we are examining a pigment that fundamentally alters plant behavior. It is phytochrome. Just as in botanical history, you knew about phytochrome effects before we discussed the identity and functions of this critical pigment. Your seed germination laboratory exercise with lettuce demonstrates a classic phytochrome effect. The seeds germinate better in red light and fail in far-red light compared to control seeds in kept in darkness. Here is a figure to extend what you observed in lab... Lettuce seeds kept in the dark germinate at low frequency. Seeds kept in the dark but briefly exposed, after imbibing water, to red light results in considerable germination. Seeds kept in the dark but briefly exposed, after imbibing water, to far-red light results in virtually no germination. Seeds kept in the dark but briefly exposed, after imbibing water, to red light and then briefly exposed to far-red light results in virtually no germination. The FR exposure appears to reverse the R response. Seeds kept in the dark but briefly exposed, after imbibing water, to far-red light and then briefly exposed to red light results in considerable germination. The R exposure appears to reverse the FR response. Lettuce seeds kept in the dark but exposed, after imbibing water, to any sequence of red and far-red light ending in FR, results in very low germination. -

Physiological Functions of Phytochromes in Tomato: a Study Using Photomorphogenic Mutants
Physiologicalfunction so fphytochrome si ntomato : astud yusin gphotomorphogeni c mutants Fysiologische functies van fytochromen in tomaat: een studie gebruikmakend van fotomorfogenetische mutanten Promotor: dr.W.J . Vredenberg hoogleraar in de plantenfysiologie metbijzonder e aandacht voor de fysische aspecten Co-promotor: dr. R.E. Kendrick universitair hoofddocent bij devakgroe p Plantenfysiologie pt,^oM\^, LeonardusHubertu s Joseph Kerckhoffs Physiologicalfunction so fphytochrome si ntomato : a study using photomorphogenic mutants Proefschrift ter verkrijging van de graad van doctor op gezag van derecto r magnificus van de Landbouwuniversiteit Wageningen, dr. CM. Karssen, in het openbaar te verdedigen op vrijdag 20 december 1996 des namiddags te vier uur in deAula . isn 31,11(02 This research was supported by a grant from the Foundation for Life Sciences (SLW), formerly the Foundation for Biological Research (BION), which is subsidized by the Netherlands Organization for Scientific Research (NWO). Cover design: Allex B. Haasdijk BI3UOTHEEK LANDBOuwuNiv;:R-rnrr CIP-data Koninklijke Bibliotheek, Den Haag Kerckhoffs, L.H.J. Physiological functions of phytochromes in tomato: astud y using photomorphogenic mutants/ L.H.J . Kerckhoffs. - [S.l. :s.n. ] ThesisWageningen .- Wit h ref. -wit h summary inDutch . ISBN90-5485-627- 0 Subject headings: photomorphogenesis / phytochrome / mutants / plant physiology / Lycopersicon esculentum ^o%?o\,z-^g Stellingen 1. De far-red light-insensitive (fri) entemporaril y redlight-insensitiv e (tri) mutanteni n tomaatzij n respectievelijk fytochroom Ae nfytochroo m Bl mutanten. 2. Inmonochromatisc hroo dlich tfungere n zowelfytochroo m Aal sB 1al shoofdrolspeler s in de anthocyaanbiosynthese in zaailingen van tomaat, terwijl micro-injectie studies alleenee nro lvoo rfytochroo m Atoekennen . Dit proefschift. -

A Closer Look at Far-Red Radiation Far-Red Radiation — Often Called Far-Red Light — Can Be Defined As Photons with Wavelengths from 700 to 800 Nanometers (Nm)
technically speaking BY ERIK RUNKLE A Closer Look at Far-Red Radiation Far-red radiation — often called far-red light — can be defined as photons with wavelengths from 700 to 800 nanometers (nm). Humans can barely see far-red radiation because it is at 1.0 the edge of our eye’s visual sensitivity to light quality. Figure Relative quantum Far-red 1 shows the far-red waveband (to the far right) relative to the 0.8 efficiency radiation spectrum of light that controls growth and development of plants (300 to 800 nm). The luminous efficiency 0.6 curve (the dashed line) represents the sensitivity of Luminous PR PFR 0.4 our eyes to light quality. As that curve illustrates, we efficiency perceive yellow light very well while we perceive the value Relative same quantity of blue or red light as less bright while 0.2 far-red appears extremely dim. Far-red promotes extension growth. Although we 0.0 can barely see far-red radiation, it has a major effect 300 400 500 600 700 800 on extension growth, meaning it influences the size of Wavelength (nm) leaves, the length of stems, and ultimately the height of Higher Lower plants. One of the ways plants perceive light quality is Energy of radiation through the pigment phytochrome, which exists in two different forms. One form is called the red-absorbing Figure 1. Far-red radiation is barely visible to us (as shown by the luminous efficiency curve) but can have big impacts on flowering form (abbreviated as PR) and the other is the far- and extension growth of plants.