CMS Snapshot December 10-17, 2020 (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Daily Unemployment Compensation Data
DISTRICT OF COLUMBIA DOES DISTRICT OF COLUMBIA DEPARTMENT OF DAILY UNEMPLOYMENT EMPLOYMENT SERVICES COMPENSATION DATA Preliminary numbers as of March 4, 2021.* Telephone Date Online Claims Daily Total Running Total Claims March 13, 2020 310 105 415 415 March 14, 2020 213 213 628 March 15, 2020 410 410 1,038 March 16, 2020 1,599 158 1,757 2,795 March 17, 2020 2,541 219 2,760 5,555 March 18, 2020 2,740 187 2,927 8,482 March 19, 2020 2,586 216 2,802 11,284 March 20, 2020 2,726 205 2,931 14,215 March 21, 2020 1,466 1,466 15,681 March 22, 2020 1,240 1,240 16,921 March 23, 2020 2,516 296 2,812 19,733 March 24, 2020 2,156 236 2.392 22,125 March 25, 2020 2,684 176 2,860 24,985 March 26, 2020 2,842 148 2,990 27,975 March 27, 2020 2,642 157 2,799 30,774 March 28, 2020 1,666 25 1,691 32,465 March 29, 2020 1,547 1,547 34,012 March 30, 2020 2,831 186 3,017 37,029 March, 31, 2020 2,878 186 3,064 40,093 April 1, 2020 2,569 186 2,765 42,858 April 2, 2020 2,499 150 2,649 45,507 April 3, 2020 2,071 300 2,371 47,878 April 4, 2020 1,067 14 1,081 48,959 April 5, 2020 1,020 1,020 49,979 April 6, 2020 2,098 155 2,253 52,232 April 7, 2020 1,642 143 1,715 54,017 April 8, 2020 1,486 142 1,628 55,645 *Recalculated and updated daily DISTRICT OF COLUMBIA Telephone DODISTRICT OF CEOLUMBIASDate Online Claims Daily Total Running Total DEPARTMENT OF DAILY UNEMPLOYMENTClaims EMPLOYMENT SERVICES April 9, 2020 1,604 111 1,715 57,360 April 10, 2020 COMPENSATION1,461 119 1,580 DATA58,940 April 11, 2020 763 14 777 59,717 April 12, 2020 698 698 60,415 April 13, 2020 1,499 104 -
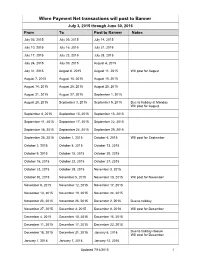
Transactions Posted to Pathway
When Payment Net transactions will post to Banner July 3, 2015 through June 30, 2016 From To Post to Banner Notes July 03, 2015 July 09, 2015 July 14, 2015 July 10, 2015 July 16, 2015 July 21, 2015 July 17, 2015 July 23, 2015 July 28, 2015 July 24, 2015 July 30, 2015 August 4, 2015 July 31, 2015 August 6, 2015 August 11, 2015 Will post for August August 7, 2015 August 13, 2015 August 18, 2015 August 14, 2015 August 20, 2015 August 25, 2015 August 21, 2015 August 27, 2015 September 1, 2015 August 28, 2015 September 3, 2015 September 9, 2015 Due to holiday at Monday Will post for August September 4, 2015 September 10, 2015 September 15, 2015 September 11, 2015 September 17, 2015 September 22, 2015 September 18, 2015 September 24, 2015 September 29, 2015 September 25, 2015 October 1, 2015 October 6, 2015 Will post for September October 2, 2015 October 8, 2015 October 13, 2015 October 9, 2015 October 15, 2015 October 20, 2015 October 16, 2015 October 22, 2015 October 27, 2015 October 23, 2015 October 29, 2015 November 3, 2015 October 30, 2015 November 5, 2015 November 10, 2015 Will post for November November 6, 2015 November 12, 2015 November 17, 2015 November 13, 2015 November 19, 2015 November 24, 2015 November 20, 2015 November 26, 2015 December 2, 2015 Due to holiday November 27, 2015 December 3, 2015 December 8, 2018 Will post for December December 4, 2015 December 10, 2015 December 15, 2015 December 11, 2015 December 17, 2015 December 22, 2015 December 18, 2015 December 31, 2015 January 6, 2016 Due to holiday closure Will post for December -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

Fall 2020 Calendar (2021-1)
FALL 2020 CALENDAR (2021-1) Polk State College 2021-1 16-Week Session FASTRACK 1 FASTRACK 2 12-Week Session Session Dates 8/17/20 - 12/9/20 8/17/20 - 10/12/20 10/14/20 - 12/9/20 9/14/20 - 12/9/20 Priority Registration - Students with 50% Complete Monday, April 6, 2020 Monday, April 6, 2020 Monday, April 6, 2020 Monday, April 6, 2020 Registration for Current Students Enrolled in 2020-1 or 2020-2 (including Dual Enrolled) Monday, April 13, 2020 Monday, April 13, 2020 Monday, April 13, 2020 Monday, April 13, 2020 Open Registration for All Students (including new Dual Enrolled students) Monday, June 1, 2020 Monday, June 1, 2020 Monday, June 1, 2020 Monday, June 1, 2020 Faculty Work Days Wed., 8/12/20 - Fri., 8/14/20 Wed., 8/12/20 - Fri., 8/14/20 Tuition Payment Plan (TPP) Enrollment 5/18/20 - 8/17/20 5/18/20 - 8/17/20 5/18/20 - 8/17/20 5/18/20 - 8/17/20 Last Day to Increase TPP Balance Monday, August 17, 2020 Monday, August 17, 2020 Monday, August 17, 2020 Monday, August 17, 2020 Financial Aid Bookstore Purchase Dates 8/7/20 - 8/24/20 8/7/20 - 8/24/20 10/4/20 - 10/21/20 9/4/20 - 9/21/20 - Error-Free FAFSA, Financial Aid Guaranteed Processing Deadline Friday, July 24, 2020 Friday, July 24, 2020 Meeting SAP, Admission Application 100% Complete Fall Convocation for Faculty and Staff Wednesday, August 12, 2020 Wednesday, August 12, 2020 First Flight Freshman Welcome Friday, August 7, 2020 Friday, August 7, 2020 Friday, August 7, 2020 Friday, August 7, 2020 First Flight Freshman Welcome Friday, August 14, 2020 Friday, August 14, 2020 Friday, August -

The Clearing House Meeting
Meeting Between Staff of the Federal Reserve Board and Representatives of The Clearing House December 10, 2020 Participants: Justyna Bolter, Jess Cheng, Alex Cordero, Lacy Douglas, Elena Falcettoni, Susan Foley, Mark Manuszak, Stephanie Martin, Zach Proom, Larkin Turman, Matthew West, and Krzysztof Wozniak (Federal Reserve Board) Philip Keitel and Rob Hunter (The Clearing House); Matthew Kane (JPMorgan Chase Bank, N.A.); Kelvin Chen and Jana Roemmich (Capital One); Duncan Douglass (Alston & Bird) Summary: Representatives from The Clearing House met with Federal Reserve Board staff to discuss their concerns about the small issuer exemption in Regulation II as it relates to partnerships between financial technology firms and banks. Attachment October 23, 2020 VIA EMAIL Matthew J. Eichner [email protected] Director — Division of Reserve Bank Operations and Payment Systems Board of Governors of the Federal Reserve System 20th Street and Constitution Avenue NW Washington, D.C. 20551 Re: Regulation II Circumvention & Recommended Actions Dear Mr. Eichner: The Clearing House Association, L.L.C.1 (“The Clearing House”) respectfully submits to the Board of Governors of the Federal Reserve System (the “Board”) the following observations for the Board’s consideration in exercising its rulemaking authority pursuant to the debit interchange fee restrictions prescribed by the Dodd-Frank Wall Street Reform and Consumer Protection Act (“Dodd-Frank Act”), as contained in Section 920 to the Electronic Fund Transfer Act (“EFTA”), and implemented -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -

Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

Due Date Chart 201803281304173331.Xlsx
Special Event Permit Application Due Date Chart for Events from January 1, 2019 - June 30, 2020 If due date lands on a Saturday or Sunday, the due date is moved to the next business day Event Date 30 Calendar days 90 Calendar Days Tuesday, January 01, 2019 Sunday, December 02, 2018 Wednesday, October 03, 2018 Wednesday, January 02, 2019 Monday, December 03, 2018 Thursday, October 04, 2018 Thursday, January 03, 2019 Tuesday, December 04, 2018 Friday, October 05, 2018 Friday, January 04, 2019 Wednesday, December 05, 2018 Saturday, October 06, 2018 Saturday, January 05, 2019 Thursday, December 06, 2018 Sunday, October 07, 2018 Sunday, January 06, 2019 Friday, December 07, 2018 Monday, October 08, 2018 Monday, January 07, 2019 Saturday, December 08, 2018 Tuesday, October 09, 2018 Tuesday, January 08, 2019 Sunday, December 09, 2018 Wednesday, October 10, 2018 Wednesday, January 09, 2019 Monday, December 10, 2018 Thursday, October 11, 2018 Thursday, January 10, 2019 Tuesday, December 11, 2018 Friday, October 12, 2018 Friday, January 11, 2019 Wednesday, December 12, 2018 Saturday, October 13, 2018 Saturday, January 12, 2019 Thursday, December 13, 2018 Sunday, October 14, 2018 Sunday, January 13, 2019 Friday, December 14, 2018 Monday, October 15, 2018 Monday, January 14, 2019 Saturday, December 15, 2018 Tuesday, October 16, 2018 2019 Tuesday, January 15, 2019 Sunday, December 16, 2018 Wednesday, October 17, 2018 Wednesday, January 16, 2019 Monday, December 17, 2018 Thursday, October 18, 2018 Thursday, January 17, 2019 Tuesday, December 18, 2018 -

FDA Briefing Document: Pfizer-Biontech COVID-19 Vaccine
Vaccines and Related Biological Products Advisory Committee Meeting December 10, 2020 FDA Briefing Document Pfizer-BioNTech COVID-19 Vaccine Sponsor: Pfizer and BioNTech Table of Contents List of Tables ............................................................................................................................. 3 List of Figures ............................................................................................................................ 4 Glossary..................................................................................................................................... 5 1. Executive Summary ............................................................................................................... 6 2. Background ............................................................................................................................ 7 2.1. SARS-CoV-2 Pandemic ................................................................................................ 7 2.2. EUA Request for the Pfizer and BioNTech COVID-19 Vaccine BNT162b2 .................... 8 2.3. U.S. Requirements to Support Issuance of an EUA for a Biological Product ........................................................................................................................... 8 2.4. Applicable Guidance for Industry ................................................................................... 9 2.5. Safety and Effectiveness Information Needed to Support an EUA ................................. 9 2.6. Continuation -

Federal Register/Vol. 85, No. 238/Thursday, December 10, 2020
79742 Federal Register / Vol. 85, No. 238 / Thursday, December 10, 2020 / Rules and Regulations DEPARTMENT OF TRANSPORTATION service animals that one passenger can such discrimination. The statute bring onboard an aircraft to two service similarly does not specify how the Office of the Secretary animals. Department should regulate with The final rule also allows airlines to respect to these issues. In addition to 14 CFR Part 382 require passengers with a disability the ACAA, the Department’s authority traveling with a service animal to to regulate nondiscrimination in airline [Docket No. DOT–OST–2018–0068] complete and submit to the airline a service on the basis of disability is based RIN No. 2105–AE63 form, developed by DOT, attesting to the in the Department’s rulemaking animal’s training and good behavior, authority under 49 U.S.C. 40113, which Traveling by Air With Service Animals and certifying the animal’s good health. states that the Department may take For flight segments of eight hours or action that it considers necessary to AGENCY: Office of the Secretary (OST), more, the rule allows airlines to require carry out this part, including prescribing U.S. Department of Transportation passengers to complete and submit a regulations. (DOT). DOT form attesting that the animal has The current rulemaking has presented ACTION: Final rule. the ability either not to relieve itself on questions about how the ACAA is a long flight or to relieve itself in a reasonably interpreted and applied to SUMMARY: The U.S. Department of sanitary manner. In addition, this final require airlines to accommodate the Transportation (Department or DOT) is rule allows airlines to require a service needs of individual passengers whose issuing a final rule to amend the animal user to provide these forms up physical or mental disability Department’s Air Carrier Access Act to 48 hours in advance of the date of necessitates the assistance of a service (ACAA) regulation on the transport of travel if the passenger’s reservation was animal in air transportation. -

Date of Close Contact Exposure
Date of Close Contact Exposure 7 days 10 days 14 days Monday, November 16, 2020 Tuesday, November 24, 2020 Friday, November 27, 2020 Tuesday, December 1, 2020 Tuesday, November 17, 2020 Wednesday, November 25, 2020 Saturday, November 28, 2020 Wednesday, December 2, 2020 Wednesday, November 18, 2020 Thursday, November 26, 2020 Sunday, November 29, 2020 Thursday, December 3, 2020 Thursday, November 19, 2020 Friday, November 27, 2020 Monday, November 30, 2020 Friday, December 4, 2020 Friday, November 20, 2020 Saturday, November 28, 2020 Tuesday, December 1, 2020 Saturday, December 5, 2020 Saturday, November 21, 2020 Sunday, November 29, 2020 Wednesday, December 2, 2020 Sunday, December 6, 2020 Sunday, November 22, 2020 Monday, November 30, 2020 Thursday, December 3, 2020 Monday, December 7, 2020 Monday, November 23, 2020 Tuesday, December 1, 2020 Friday, December 4, 2020 Tuesday, December 8, 2020 Tuesday, November 24, 2020 Wednesday, December 2, 2020 Saturday, December 5, 2020 Wednesday, December 9, 2020 Wednesday, November 25, 2020 Thursday, December 3, 2020 Sunday, December 6, 2020 Thursday, December 10, 2020 Thursday, November 26, 2020 Friday, December 4, 2020 Monday, December 7, 2020 Friday, December 11, 2020 Friday, November 27, 2020 Saturday, December 5, 2020 Tuesday, December 8, 2020 Saturday, December 12, 2020 Saturday, November 28, 2020 Sunday, December 6, 2020 Wednesday, December 9, 2020 Sunday, December 13, 2020 Sunday, November 29, 2020 Monday, December 7, 2020 Thursday, December 10, 2020 Monday, December 14, 2020 Monday, November -

Dates for Student-Run Credit Unions 15-16 Northville Schools Northern Schools Novi & Livonia Schools
Dates for Student-Run Credit Unions 15-16 Northville Schools Northern Schools Novi & Livonia Schools Plymouth-Canton Elementary Schools: Bentley Bird Dodson Eriksson Credit Union Credit Union Credit Union Credit Union Tuesdays: Thursdays: October 6 Wednesdays: Tuesdays: October 13 October 20 October 7 October 20 October 27 November 10 October 28 November 10 November 17 November 24 November 18 December 1 December 8 December 8 December 16 December 15 January 5 January 12 January 5 January 6 January 19 January 26 January 19 January 27 February 2 February 9 February 2 February 10 February 23 March 1 February 23 March 2 March 8 March 15 March 8 March 23 March 22 April 5 March 22 April 13 April 12 April 5 May 4 April 19 April 26 April 19 May 25 May 3 May 10 May 17 May 3 Farrand Field Gallimore Hoben Credit Union Credit Union Credit Union Credit Union Wednesdays: Tuesdays: Thursdays: Thursdays: October 21 October 20 October 8 October 15 November 11 November 17 October 22 November 12 December 9 December 1 November 12 December 10 January 6 December 15 December 3 January 14 February 3 January 12 January 7 February 11 February 24 January 26 March 10 March 16 February 4 February 9 April 14 April 6 February 25 February 23 May 12 May 4 March 8 March 10 March 22 April 7 April 12 April 28 April 26 May 12 May 10 June 2 Hulsing Isbister Credit Miller Smith Credit Union Union Credit Union Credit Union Thursdays: Wednesdays: October 1 Thursdays: Thursdays: September 30 October 15 October 8 October 1 October 14 November 19 November 5 October 22 October 28