A Geochemical Study of Four Prehistoric Quarries in Oaxaca, Mexico
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
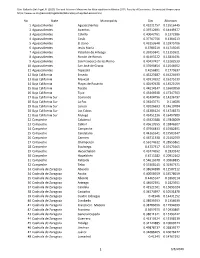
No. State Municipality Gini Atkinson 1 Aguascalientes Aguascalientes
Cite: Gallardo Del Angel, R. (2020) Gini and Atkinson Measures for Municipalities in Mexico 2015. Faculty of Economics. Universidad Veracruzana. https://www.uv.mx/personal/rogallardo/laboratory-of-applied-economics/ No. State Municipality Gini Atkinson 1Aguascalientes Aguascalientes 0.43221757 0.15516445 2Aguascalientes Asientos 0.39712691 0.14449377 3Aguascalientes Calvillo 0.40042761 0.1372586 4Aguascalientes Cosío 0.37767756 0.1304213 5Aguascalientes El Llano 0.43253648 0.15975706 6Aguascalientes Jesús María 0.3780219 0.11715045 7Aguascalientes Pabellón de Arteaga 0.39355841 0.13155921 8Aguascalientes Rincón de Romos 0.41403222 0.15831631 9Aguascalientes San Francisco de los Romo 0.40437427 0.15282539 10Aguascalientes San José de Gracia 0.37699854 0.12548952 11Aguascalientes Tepezalá 0.4256891 0.1779637 12Baja California Enseda 0.43223187 0.16224693 13Baja California Mexicali 0.43914592 0.16275139 14Baja California Playas de Rosarito 0.42097628 0.14521059 15Baja California Tecate 0.44214147 0.16600958 16Baja California Tijua 0.45446938 0.17347503 17Baja California Sur Comondú 0.41404796 0.14236797 18Baja California Sur La Paz 0.36343771 0.114026 19Baja California Sur Loreto 0.42026693 0.14610784 20Baja California Sur Los Cabos 0.41386124 0.14718573 21Baja California Sur Mulegé 0.42451236 0.16497989 22Campeche Calakmul 0.45923388 0.17848009 23Campeche Calkiní 0.43610555 0.15846507 24Campeche Campeche 0.47966433 0.19382891 25Campeche Candelaria 0.44265541 0.17503347 26Campeche Carmen 0.46711338 0.21462959 27Campeche Champotón 0.56174612 -

Sources and Resources/ Fuentes Y Recursos
ST. FRANCIS AND THE AMERICAS/ SAN FRANCISCO Y LAS AMÉRICAS: Sources and Resources/ Fuentes y Recursos Compiled by Gary Francisco Keller 1 Table of Contents Sources and Resources/Fuentes y Recursos .................................................. 6 CONTROLLABLE PRIMARY DIGITAL RESOURCES 6 Multimedia Compilation of Digital and Traditional Resources ........................ 11 PRIMARY RESOURCES 11 Multimedia Digital Resources ..................................................................... 13 AGGREGATORS OF CONTROLLABLE DIGITAL RESOURCES 13 ARCHIVES WORLDWIDE 13 Controllable Primary Digital Resources 15 European 15 Mexicano (Nahuatl) Related 16 Codices 16 Devotional Materials 20 Legal Documents 20 Maps 21 Various 22 Maya Related 22 Codices 22 Miscellanies 23 Mixtec Related 23 Otomi Related 24 Zapotec Related 24 Other Mesoamerican 24 Latin American, Colonial (EUROPEAN LANGUAGES) 25 PRIMARY RESOURCES IN PRINTED FORM 25 European 25 Colonial Latin American (GENERAL) 26 Codices 26 2 Historical Documents 26 Various 37 Mexicano (Nahautl) Related 38 Codices 38 Lienzo de Tlaxcala 44 Other Lienzos, Mapas, Tiras and Related 45 Linguistic Works 46 Literary Documents 46 Maps 47 Maya Related 48 Mixtec Related 56 Otomí Related 58 (SPREAD OUT NORTH OF MEXICO CITY, ALSO HIDALGO CLOSELY ASSOCIATED WITH THE OTOMÍ) Tarasco Related 59 (CLOSELY ASSOCIATED WITH MICHOACÁN. CAPITAL: TZINTZUNRZAN, LANGUAGE: PURÉPECHA) Zapotec Related 61 Other Mesoamerican 61 Latin American, Colonial (EUROPEAN LANGUAGES) 61 FRANCISCAN AND GENERAL CHRISTIAN DISCOURSE IN NATIVE -

Maquetación HA 25/05/2018 14:23 Página 33
RHA75__Maquetación HA 25/05/2018 14:23 Página 33 Historia Agraria, 75 I Agosto 2018 I pp. 33-68 I DOI 10.26882/histagrar.075e03g © 2018 SEHA New crops, new landscapes and new socio-political relationships in the cañada de Yosotiche (Mixteca region, Oaxaca, Mexico), 16th-18th centuries MARTA MARTÍN GABALDÓN KEYWORDS: ecological complementarity, yuhuitayu, sugar cane, Mixteca region. JEL CODES: N56, N96, O33, Q15. ur aim is to determine continuities and changes in the cañada of Yosotiche environment since the introduction by Spanish conquerors and settlers of new Ocrops, especially sugarcane. A study of the biological modifications of a par- ticular ecosystem allows inferences on changes and continuities in socio-political rela- tions. This particular case study contributes to a discussion of the general model of Mix- tec political territoriality. The methodology applied here involves a convergence that integrates the analysis of historical documents, archaeological data, fieldwork and anth- ropological information, along with discoveries made by earlier research. It offers in- sight into occupational dynamics and their ties to the political, administrative, econo- mic and social structures within the cañada during colonial times. The introduction of foreign crops produced changes in the ecological complemen- tarity system practiced by the villages that possessed lands in the cañada, consequently modifying the labour relations of the inhabitants. An analysis of this situation reveals the singular status of the lands owned by Tlaxiaco, which seemingly fit the regulations dictated by the Laws of the Indies but, in essence, meant the continuity of pre-Hispa- nic traditions. 33 RHA75__Maquetación HA 25/05/2018 14:23 Página 34 Marta Martín Gabaldón Nuevos cultivos, nuevos paisajes y nuevas relaciones político-sociales en la cañada de Yosotiche (región mixteca, Oaxaca, México), siglos XVI-XVIII PALABRAS CLAVE: complementariedad ecológica, yuhuitayu, caña de azúcar, región mixteca. -

Determining the Vulnerability of Mexican Pine Forests to Bark Beetles of the Genus Dendroctonus Erichson (Coleoptera: Curculionidae: Scolytinae)
Determining the vulnerability of Mexican pine forests to bark beetles of the genus Dendroctonus Erichson (Coleoptera: Curculionidae: Scolytinae) Y. Salinas-Morenoa,*, A. Agerb, C.F.Vargasa,J.L. Hayesc, G. Zunigaa ABSTRACT Bark beetles of the genus Dendroctonus are natural inhabitants of forests; under particular conditions some species of this genus can cause large-scale tree mortality. However, only in recent decades has pri- ority been given to the comprehensive study of these insects in Mexico. Mexico possesses high ecological diversity in Dendroctonus-Pinus associations. The geographic coexistence of 12 Dendroctonus species suggests greater vulnerability or threat of tree mortality relative to other areas. We use a biogeographic strategy to identify and rank the areas most vulnerable to tree mortality caused by bark beetles in Mex- ico. We aim to define the areas that might experience high impact by these insects and also to provide a geographic database useful to forest resource management and conservation policies in Mexico. Using collection records of bark beetles and pines, we develop a quantitative estimate of the threat of beetle infestation of forest areas based on factors including pine and beetle species density, host preference and level of mortality caused by beetle species. A quantitative estimate of forest area vulnerability, the Bark Beetle Threat Index (BBTI) was calculated. Despite the vast area of geographic coincidence of Pinus and Dendroctonus in Mexico, the regions of highest bark beetle pressure are restricted to small zones within some mountain systems. The region that has been most affected by this insect group during the past hundred years is the Transverse Volcanic Belt, followed by the Sierra Madre Occidental and Sierra Madre del Sur. -

Curriculum Vitae John K. Chance
Curriculum Vitae John K. Chance Present Position Professor of Anthropology School of Human Evolution and Social Change Arizona State University Tempe, Arizona 85287-2402 Telephone: (480)-965-4843 Fax: (480)-965-7671 Email: [email protected] Education 1967 A.B., University of Pennsylvania, major in Anthropology. 1971 A.M. in Anthropology, University of Illinois at Urbana-Champaign. 1974 Ph.D. in Anthropology, University of Illinois at Urbana-Champaign. Employment History 1968, Teaching Assistant, Department of Anthropology, 1973-74 University of Illinois at Urbana-Champaign. 1974-80 Assistant Professor, Department of Anthropology, Lawrence University, Appleton, Wisconsin. 1974-75 Chair, Department of Anthropology, Lawrence University. 1979 Acting Chair, Department of Anthropology, Spring, Lawrence University. 1980-85 Associate Professor and Chair, Department of Anthropology, University of Denver. 1985-86 Professor and Chair, Departamento de Antropología y Sociología, Universidad de las Américas, Puebla, Mexico. 1986-87 Visiting Associate Professor, Department of Anthropology, and Assistant Director, Center for Latin American and Caribbean Studies, University of Illinois at Urbana-Champaign. 1987-91 Associate Professor of Anthropology, Arizona State University. 1991-present Professor of Anthropology, Arizona State University. 1995-99 Associate Chair, Department of Anthropology, Arizona State University 1998-99 Acting Chair, Department of Anthropology, Arizona State University; June and July 1998, February-April, 1999 1999-2003 Chair, Department of Anthropology, Arizona State University Language Proficiency Complete fluency in Spanish, including paleography; reading fluency in French and some in Classical Nahuatl. Major Research and Teaching Interests Sociocultural anthropology, ethnohistory, political economy, social inequality; Mesoamerica. Predoctoral Scholarships and Fellowships 1968-71 National Defense Foreign Language Title VI predoctoralfellowship, University of Illinois at Urbana-Champaign. -

Movilidad Y Desarrollo Regional En Oaxaca
ISSN 0188-7297 Certificado en ISO 9001:2000‡ “IMT, 20 años generando conocimientos y tecnologías para el desarrollo del transporte en México” MOVILIDAD Y DESARROLLO REGIONAL EN OAXACA VOL1: REGIONALIZACIÓN Y ENCUESTA DE ORIGÉN Y DESTINO Salvador Hernández García Martha Lelis Zaragoza Manuel Alonso Gutiérrez Víctor Manuel Islas Rivera Guillermo Torres Vargas Publicación Técnica No 305 Sanfandila, Qro 2006 SECRETARIA DE COMUNICACIONES Y TRANSPORTES INSTITUTO MEXICANO DEL TRANSPORTE Movilidad y desarrollo regional en oaxaca. Vol 1: Regionalización y encuesta de origén y destino Publicación Técnica No 305 Sanfandila, Qro 2006 Esta investigación fue realizada en el Instituto Mexicano del Transporte por Salvador Hernández García, Víctor M. Islas Rivera y Guillermo Torres Vargas de la Coordinación de Economía de los Transportes y Desarrollo Regional, así como por Martha Lelis Zaragoza de la Coordinación de Ingeniería Estructural, Formación Posprofesional y Telemática. El trabajo de campo y su correspondiente informe fue conducido por el Ing. Manuel Alonso Gutiérrez del CIIDIR-IPN de Oaxaca. Índice Resumen III Abstract V Resumen ejecutivo VII 1 Introducción 1 2 Situación actual de Oaxaca 5 2.1 Situación socioeconómica 5 2.1.1 Localización geográfica 5 2.1.2 Organización política 6 2.1.3 Evolución económica y nivel de desarrollo 7 2.1.4 Distribución demográfica y pobreza en Oaxaca 15 2.2 Situación del transporte en Oaxaca 17 2.2.1 Infraestructura carretera 17 2.2.2 Ferrocarriles 21 2.2.3 Puertos 22 2.2.4 Aeropuertos 22 3 Regionalización del estado -

The Economy of Oaxaca Decomposed
Georgia Southern University Digital Commons@Georgia Southern University Honors Program Theses 2015 The conomE y of Oaxaca Decomposed Albert Codina Sala Georgia Southern University Follow this and additional works at: https://digitalcommons.georgiasouthern.edu/honors-theses Part of the Growth and Development Commons, Income Distribution Commons, International Economics Commons, Macroeconomics Commons, and the Regional Economics Commons Recommended Citation Codina Sala, Albert, "The cE onomy of Oaxaca Decomposed" (2015). University Honors Program Theses. 89. https://digitalcommons.georgiasouthern.edu/honors-theses/89 This thesis (open access) is brought to you for free and open access by Digital Commons@Georgia Southern. It has been accepted for inclusion in University Honors Program Theses by an authorized administrator of Digital Commons@Georgia Southern. For more information, please contact [email protected]. The Economy of Oaxaca Decomposed An Honors Thesis submitted in partial fulfillment of the requirements for Honors in Department of Finance and Economics. By Albert Codina Sala Under the mentorship of Dr. Gregory Brock ABSTRACT We analyze the internal economy of Oaxaca State in southern Mexico across regions, districts and municipalities from 1999 to 2009. Using the concept of economic convergence, we find mixed evidence for poorer areas catching up with richer areas during a single decade of economic growth. Indeed, some poorer regions thanks to negative growth have actually diverged away from wealthier areas. Keywords: Oaxaca, Mexico, Beta Convergence, Sigma Convergence Thesis Mentor: _____________________ Dr. Gregory Brock Honors Director: _____________________ Dr. Steven Engel April 2015 College of Business Administration University Honors Program Georgia Southern University Acknowledgements The first person I would like to thank is my research mentor Dr. -

Fuzzy Modeling of Migration from the State of Oaxaca, Mexico
Fuzzy Modeling of Migration from the State of Oaxaca, Mexico Anais Vermonden and Carlos Gay Programa de Investigación en Cambio Climático, Universidad Nacional Autónoma de México, CU, Mexico Keywords: Fuzzy Logic, Migration, Oaxaca, Mexico, ANFIS. Abstract: This study shows an important innovation with the use of fuzzy logic to develop models on the migration factors occurring in the state of Oaxaca, México, since fuzzy logic has not been applied in this field. Migration is a complex system as individuals make their own decision to migrate. The major factors causing migration are: higher employment in the primary sector, high grades of unemployment, high marginalization index, small communities, soil degradation, violence and remittance received. Another tendency shown in these models is that municipalities in Oaxaca with greater levels of education are having higher migration levels due to the lack of opportunities to continue studies or well-paid jobs. Climate change may impose greater movement of people as it can worsen the already precarious soil situation. Even if the models present some error in the calculation of the migration index, it made clear what other variables should be included to show the impacts of climate change on migration. 1 INTRODUCTION 2013). There are a number of causes for the migration Oaxaca is located in southwest Mexico; it is divided from Oaxaca, and some of the most significant are into 570 municipalities. Oaxaca is one of the poorest lack of economic development, need for states with 61.9% of the population living under the diversification of income (remittance), ecological poverty line and, has one of the highest rates of rural deterioration, lack of educational opportunities, migration. -

Acuerdo Del Comité Ejecutivo Nacional Y La Comisión Nacional De
ACUERDO DEL COMITÉ EJECUTIVO NACIONAL Y LA COMISIÓN NACIONAL DE ELECCIONES POR EL CUAL SE CANCELAN LAS ASAMBLEAS MUNICIPALES ELECTORALES EN DIVERSOS ESTADOS DE LA REPÚBLICA DENTRO DEL PROCESO DE SELECCIÓN INTERNA DE CANDIDATOS 2017 - 2018 Con fundamento en lo dispuesto en los artículos 14, 16 y 41, Base I, de la Constitución Política de los Estados Unidos Mexicanos; en relación con los diversos 3 y 5, párrafo 2, de la Ley General de Partidos Políticos; 4°, 5°, 13°, 14° bis, 24° último párrafo, 42°, 43°, 44°, 45°, 46°, y demás relativos y aplicables del Estatuto de MORENA; y lo previsto en el Segundo Transitorio de la Convocatoria al proceso de selección interna de candidatos/as para ser postulados/as en los procesos electorales federales y locales 2017 – 2018; y CONSIDERANDO I. – Que el Comité Ejecutivo Nacional y la Comisión Nacional de Elecciones son competentes para emitir el presente acuerdo, en términos de las atribuciones que les confieren los artículos 38°, 44°, inciso w), 46°, y demás relativos y aplicables del Estatuto de MORENA; y el Segundo Transitorio de la Convocatoria al proceso de selección interna de candidatos/as para ser postulados/as en los procesos electorales federales y locales 2017 – 2018. II. – Que de acuerdo con las Bases Operativas y sus respectivas fe de erratas de los Estados de CHIAPAS, CHIHUAHUA; COAHUILA; GUANAJUATO; GUERRERO; MICHOACÁN, JALISCO, PUEBLA, OAXACA, QUERÉTARO, SAN LUIS POTOSÍ, SONORA; TAMAULIPAS y ZACATECAS; se programó la realización de las Asambleas Municipales Electorales en dichas entidades a celebrarse entre el 7, 8 y 9 de Febrero de 2018, respectivamente. -

Evolution of the Guerrero Composite Terrane Along the Mexican Margin, from Extensional Fringing Arc to Contractional Continental Arc
Evolution of the Guerrero composite terrane along the Mexican margin, from extensional fringing arc to contractional continental arc Elena Centeno-García1,†, Cathy Busby2, Michael Busby2, and George Gehrels3 1Instituto de Geología, Universidad Nacional Autónoma de México, Avenida Universidad 3000, Ciudad Universitaria, México D.F. 04510, México 2Department of Geological Sciences, University of California, Santa Barbara, California 93106-9630, USA 3Department of Geosciences, University of Arizona, Tucson, Arizona 85721, USA ABSTRACT semblage shows a Callovian–Tithonian (ca. accreted to the edge of the continent during 163–145 Ma) peak in magmatism; extensional contractional or oblique contractional phases The western margin of Mexico is ideally unroofing began in this time frame and con- of subduction. This process can contribute sub- suited for testing two opposing models for tinued into through the next. (3) The Early stantially to the growth of a continent (Collins, the growth of continents along convergent Cretaceous extensional arc assemblage has 2002; Busby, 2004; Centeno-García et al., 2008; margins: accretion of exotic island arcs by two magmatic peaks: one in the Barremian– Collins, 2009). In some cases, renewed upper- the consumption of entire ocean basins ver- Aptian (ca. 129–123 Ma), and the other in the plate extension or oblique extension rifts or sus accretion of fringing terranes produced Albian (ca. 109 Ma). In some localities, rapid slivers these terranes off the continental margin by protracted extensional processes in the subsidence produced thick, mainly shallow- once more, in a kind of “accordion” tectonics upper plate of a single subduction zone. We marine volcano-sedimentary sections, while along the continental margin, referred to by present geologic and detrital zircon evidence at other localities, extensional unroofing of Collins (2002) as tectonic switching. -

The Taxco Epithermal Deposits, Guerrero
Geochronology of Mexican mineral deposits 357 Boletín de la Sociedad Geológica Mexicana Volumen 67, núm. 2, 2015, p. 357-366 D GEOL DA Ó E G I I C C O A S 1904 M 2004 . C EX . ICANA A C i e n A ñ o s Short Note Geochronology of Mexican mineral deposits. III: the Taxco epithermal deposits, Guerrero José L. Farfán-Panamá1,2, Antoni Camprubí3,*, Eduardo González-Partida4, Alexander Iriondo4, Enrique Gonzalez-Torres3,5 1 Unidad Académica de Ciencias de la Tierra, Universidad Autónoma de Guerrero, Ex-Hacienda de San Juan Bautista, 40323 Taxco el Viejo, Gro., México. 2 Programa de Posgrado en Ciencias de la Tierra, Universidad Nacional Autónoma de México, Boulevard Juriquilla 3001, 76230 Querétaro, Qro., México. 3 Instituto de Geología, Universidad Nacional Autónoma de México, Ciudad Universitaria, 04510, D.F., Mexico. 4 Centro de Geociencias, Universidad Nacional Autónoma de México, Boulevard Juriquilla 3001, 76230 Querétaro, Qro., Mexico. 5 Facultad de Ingeniería, Universidad Nacional Autónoma de México. Ciudad Universitaria, 04510, D.F., Mexico. * [email protected] Abstract New 40Ar/39Ar (34.96 ± 0.19 Ma) and U-Pb ages (35.44 ± 0.24 and 34.95 ± 0.37 Ma) obtained in this study for the Calavera group of dikes, which predate intermediate sulfidation epithermal mineralization in the Taxco mining district, constrain the formation of such deposits to less than 34.96 Ma (latest Eocene). These deposits might arguably have formed at ~ 33 Ma, thus coinciding in age with the La Azul fluorite deposits, within the same district. Although this age is significantly younger than previously existing estimations, the deposits at Taxco consistently cluster into a Late Eocene to Oligocene metallogenic event. -

OECD Territorial Grids
BETTER POLICIES FOR BETTER LIVES DES POLITIQUES MEILLEURES POUR UNE VIE MEILLEURE OECD Territorial grids August 2021 OECD Centre for Entrepreneurship, SMEs, Regions and Cities Contact: [email protected] 1 TABLE OF CONTENTS Introduction .................................................................................................................................................. 3 Territorial level classification ...................................................................................................................... 3 Map sources ................................................................................................................................................. 3 Map symbols ................................................................................................................................................ 4 Disclaimers .................................................................................................................................................. 4 Australia / Australie ..................................................................................................................................... 6 Austria / Autriche ......................................................................................................................................... 7 Belgium / Belgique ...................................................................................................................................... 9 Canada ......................................................................................................................................................