Newer Therapies in COPD
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dosing Time Matters
bioRxiv preprint doi: https://doi.org/10.1101/570119; this version posted March 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Dosing Time Matters 1 2,3 4,5,6 1* Marc D. Ruben , David F. Smith , Garret A. FitzGerald , and John B. Hogenesch 1 Division of Human Genetics, Center for Chronobiology, Department of Pediatrics, Cincinnati Children's Hospital Medical Center, 240 Albert Sabin Way, Cincinnati, OH, 45229 2 Divisions of Pediatric Otolaryngology and Pulmonary and Sleep Medicine, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229 3 Department of Otolaryngology-Head and Neck Surgery, University of Cincinnati School of Medicine, 231 Albert Sabin Way, Cincinnati, OH, 45267 4 Department of Systems Pharmacology and Translational Therapeutics, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 5 Department of Medicine, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 6 Institute for Translational Medicine and Therapeutics (ITMAT), at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA *Corresponding Author. Email: [email protected] Abstract Trainees in medicine are taught to diagnose and administer treatment as needed; time-of-day is rarely considered. Yet accumulating evidence shows that ~half of human genes and physiologic functions follow daily rhythms. Circadian medicine aims to incorporate knowledge of these rhythms to enhance diagnosis and treatment. -

New Zealand Data Sheet
NEW ZEALAND DATA SHEET 1. PRODUCT NAME INCRUSE ELLIPTA Umeclidinium (as bromide), 62.5 micrograms, powder for inhalation 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each delivered dose (the dose leaving the mouthpiece of the inhaler) contains 55 micrograms umeclidinium (equivalent to 65 micrograms of umeclidinium bromide). This corresponds to a pre-dispensed dose of 62.5 micrograms of umeclidinium (equivalent to 74.2 micrograms umeclidinium bromide). Excipient with known effect: Each delivered dose contains approximately 12.5 mg of lactose (as monohydrate). For the full list of excipients, see section 6.1 List of excipients. 3. PHARMACEUTICAL FORM Powder for Inhalation White powder in a grey inhaler (Ellipta) with a light green mouthpiece cover and a dose counter. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Incruse Ellipta is indicated as a long-term once daily maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD). 4.2 Dose and method of administration Dose Adults Incruse Ellipta (umeclidinium 62.5 micrograms) should be taken as one inhalation once daily by the orally inhaled route. Incruse Ellipta should be taken at the same time every day. Do not use Incruse Ellipta more than once every 24 hours. Special populations Elderly population No dosage adjustment is required in patients over 65 years (see 5.2 Pharmacokinetics properties– Special patient populations). 1 Renal impairment No dosage adjustment is required in patients with renal impairment (see 5.2 Pharmacokinetics properties – Special patient populations). Hepatic impairment No dosage adjustment is required in patients with mild or moderate hepatic impairment. Incruse Ellipta has not been studied in patients with severe hepatic impairment (see 5.2 Pharmacokinetics properties– Special patient populations). -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group R01 (Nasal preparations) Table of Contents Page INTRODUCTION 5 DISCLAIMER 7 GLOSSARY OF TERMS USED IN THIS DOCUMENT 8 ACTIVE SUBSTANCES Cyclopentamine (ATC: R01AA02) 10 Ephedrine (ATC: R01AA03) 11 Phenylephrine (ATC: R01AA04) 14 Oxymetazoline (ATC: R01AA05) 16 Tetryzoline (ATC: R01AA06) 19 Xylometazoline (ATC: R01AA07) 20 Naphazoline (ATC: R01AA08) 23 Tramazoline (ATC: R01AA09) 26 Metizoline (ATC: R01AA10) 29 Tuaminoheptane (ATC: R01AA11) 30 Fenoxazoline (ATC: R01AA12) 31 Tymazoline (ATC: R01AA13) 32 Epinephrine (ATC: R01AA14) 33 Indanazoline (ATC: R01AA15) 34 Phenylephrine (ATC: R01AB01) 35 Naphazoline (ATC: R01AB02) 37 Tetryzoline (ATC: R01AB03) 39 Ephedrine (ATC: R01AB05) 40 Xylometazoline (ATC: R01AB06) 41 Oxymetazoline (ATC: R01AB07) 45 Tuaminoheptane (ATC: R01AB08) 46 Cromoglicic Acid (ATC: R01AC01) 49 2 Levocabastine (ATC: R01AC02) 51 Azelastine (ATC: R01AC03) 53 Antazoline (ATC: R01AC04) 56 Spaglumic Acid (ATC: R01AC05) 57 Thonzylamine (ATC: R01AC06) 58 Nedocromil (ATC: R01AC07) 59 Olopatadine (ATC: R01AC08) 60 Cromoglicic Acid, Combinations (ATC: R01AC51) 61 Beclometasone (ATC: R01AD01) 62 Prednisolone (ATC: R01AD02) 66 Dexamethasone (ATC: R01AD03) 67 Flunisolide (ATC: R01AD04) 68 Budesonide (ATC: R01AD05) 69 Betamethasone (ATC: R01AD06) 72 Tixocortol (ATC: R01AD07) 73 Fluticasone (ATC: R01AD08) 74 Mometasone (ATC: R01AD09) 78 Triamcinolone (ATC: R01AD11) 82 -

Intranasal Rhinitis Agents
Intranasal Rhinitis Agents Therapeutic Class Review (TCR) February 1, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. -

Early Protective Effects of Tiotropium Bromide in Patients with Airways Hyperres P O N S I V E N E S S
European Review for Medical and Pharmacological Sciences 2004; 8: 259-264 Early protective effects of tiotropium bromide in patients with airways hyperres p o n s i v e n e s s C. TERZANO, A. PETROIANNI, A. RICCI, L. D’ANTONI, L. ALLEGRA* Department of Cardiovascular and Respiratory Sciences, Respiratory Diseases Unit, Fondazione E. Lorillard Spencer Cenci, “La Sapienza” University - Rome (Italy) *Institute of Respiratory Diseases, University of Milan, Ospedale Maggiore IRCCS – Milan (Italy) Abstract. – Tiotropium is an anticholiner- Introduction gic drug for Ch ronic Obstructive Pulmonary Di sease (COPD) patients, with a peak b ron- Ti o t rop ium is a qu atern a ry amm o niu m chodilator effect observed after 1.5 to 2 hours and a long duration of action. The aim of our co ngener of atropine. It has been developed study was to quantify the early protection of a as a lon g-acting an ticho linergic bro n c h o d i l a- single dose of inhaled tiotropium against metha- tor for inhalation by patients with chronic ob- choline-induced bronchoconstriction in asthmat- s t ructive pu lmon ary d isease (COPD). Dru g ic patients with airway hyperresponsiveness. p ro p e rties o f ant icho lin ergic agent s have Ten subjects (7M, 3F), with history of asthma been well-kno wn to man y cultures for many and a baseline FEV (Forced Expiratory Volume 1 1 1 c e n t u r i e s . In the 1920s the d iscovery o f the sec) >80% of pred icted, were enrolled in the s t u d y. -

Effect of Ipratropium Bromide on Mucociliary Clearance and Pulmonary Function in Reversible Airways Obstruction
Thorax: first published as 10.1136/thx.34.4.501 on 1 August 1979. Downloaded from Thorax, 1979, 34, 501-507 Effect of ipratropium bromide on mucociliary clearance and pulmonary function in reversible airways obstruction DEMETRI PAVIA, J RODERICK M BATEMAN, NOIRIN F SHEAHAN, AND STEWART W CLARKE From the Department of Thoracic Medicine, The Royal Free Hospital, London NW3 2QG, UK ABSTRACT The effects of (a) regular use for one week and (b) a single dose of a synthetic anticholinergic (ipratropium bromide) on lung mucociliary clearance and as a bronchodilator was ascertained in a controlled, double-blind, cross-over study in 12 patients with reversible airways obstruction (mean increase in FEV1 after isoprenaline: 17%, range 10-50%). Two puffs from a metered dose inhaler of either placebo (propellants only) or drug (40 ,ug) were administered four times a day for one week (regular use), and mucociliary clearance was measured, by radioaerosol tracer, at the end of each treatment period and after a control period in which no treatment was given. On the mornings of the measurements after the placebo and drug periods one final dose (single dose) of ipratropium (40 ,ug) or placebo was given 2 5 hours before the start of the test. There was no statistically significant difference between the three mean mucociliary clearance curves (control, placebo, and drug) for the group; however, there was a significantly greater penetration towards the periphery of the lung of the tracer in the test was after drug administration compared with the other two. This increased penetration http://thorax.bmj.com/ attributed to bronchodilatation caused by the drug. -

Stable Pressurised Aerosol Solution Composition of Glycopyrronium Bromide and Formoterol Combination
(19) TZZ¥¥_T (11) EP 3 384 898 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 10.10.2018 Bulletin 2018/41 A61K 9/00 (2006.01) A61M 15/00 (2006.01) A61K 31/167 (2006.01) A61K 31/40 (2006.01) (2006.01) (2006.01) (21) Application number: 18171127.6 B65D 83/54 A61K 31/573 A61K 31/54 (2006.01) A61K 45/06 (2006.01) (2006.01) (2006.01) (22) Date of filing: 23.12.2014 A61K 9/12 A61K 31/58 A61M 39/22 (2006.01) (84) Designated Contracting States: • COPELLI, Diego AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 43100 PARMA (IT) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • DAGLI ALBERI, Massimiliano PL PT RO RS SE SI SK SM TR 43100 PARMA (IT) Designated Extension States: • USBERTI, Francesca BA ME 43100 PARMA (IT) • ZAMBELLI, Enrico (30) Priority: 30.12.2013 EP 13199784 43100 PARMA (IT) (62) Document number(s) of the earlier application(s) in (74) Representative: Bianchetti Bracco Minoja S.r.l. accordance with Art. 76 EPC: Via Plinio, 63 14825154.9 / 3 089 735 20129 Milano (IT) (71) Applicant: Chiesi Farmaceutici S.p.A. Remarks: 43100 Parma (IT) This application was filed on 08-05-2018 as a divisional application to the application mentioned (72) Inventors: under INID code 62. • BONELLI, Sauro 43100 PARMA (IT) (54) STABLE PRESSURISED AEROSOL SOLUTION COMPOSITION OF GLYCOPYRRONIUM BROMIDE AND FORMOTEROL COMBINATION (57) The invention concerns an aerosol solution lower than the limit of quantification, when stored in ac- composition intended for use with a pressurised metered celerated conditions at 25°C and 60% relative humidity dose inhaler, comprising glycopyrronium bromide and (RH) for 6 months in a can internally coated by a resin formoterol, or a salt thereof or a solvate of said salt, op- comprising a fluorinated ethylene propylene (FEP) poly- tionally in combination with one or more additional active mer. -

Hypersalivation in Children and Adults
Pharmacological Management of Hypersalivation in Children and Adults Scope: Adult patients with Parkinson’s disease, children with neurodisability, cerebral palsy, long-term ventilation with drooling, and drug-induced hypersalivation. ASSESSMENT OF SEVERITY/RESPONSE TO TREATMENT: Severity of drooling can be assessed subjectively via discussion with patients and their carers/parents and by observation. Amount of drooling can be quantified by measuring the number of bibs required per day and this can also be graded using the Thomas-Stonell and Greenberg scale: • 1 = Dry (no drooling) • 2 = Mild (moist lips) • 3 = Moderate (wet lips and chin) • 4 = Severe (damp clothing) CONSIDERATIONS FOR PRESCRIBING/TITRATION No evidence to support the use of one particular treatment over another. Drug choice is to be determined by individual patient factors. When prescribing/titrating antimuscarinic drugs to treat hypersalivation always take account of: • Coexisting conditions (for example, history of urinary retention, constipation, glaucoma, dental issues, reflux etc.) • Use of other existing medication affecting the total antimuscarinic burden • Risk of adverse effects Titrate dose upward until the desired level of dryness, side effects or maximum dose reached. Take into account the preferences of the patients and their carers/ parents, and the age range and indication covered by the marketing authorisations (see individual summaries of product characteristics, BNF or BNFc for full prescribing information). FIRST LINE DRUG TREATMENT OPTIONS FOR ADULTS -

COPD Agents Review – October 2020 Page 2 | Proprietary Information
COPD Agents Therapeutic Class Review (TCR) October 1, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. October 2020 -

New Zealand Data Sheet
NEW ZEALAND DATA SHEET 1. PRODUCT NAME TRELEGY ELLIPTA 100/62.5/25 fluticasone furoate (100 micrograms)/umeclidinium (as bromide) (62.5 micrograms)/vilanterol (as trifenatate) (25 micrograms), powder for inhalation 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each delivered dose (the dose leaving the mouthpiece of the inhaler) contains 92 micrograms fluticasone furoate, 55 micrograms umeclidinium (equivalent to 65 micrograms umeclidinium [as bromide]) and 22 micrograms vilanterol (as trifenatate). This corresponds to a pre-dispensed dose of 100 micrograms fluticasone furoate, 62.5 micrograms umeclidinium (equivalent to 74.2 micrograms umeclidinium bromide) and 25 micrograms vilanterol (as trifenatate). Excipient with known effect: Each delivered dose contains approximately 25 milligrams of lactose (as monohydrate). For the full list of excipients, see Section 6.1 List of excipients. 3. PHARMACEUTICAL FORM Powder for inhalation. White powder in a light grey inhaler (Ellipta) with a beige mouthpiece cover and a dose counter. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications TRELEGY ELLIPTA is indicated for the maintenance treatment of adults with moderate to severe chronic obstructive pulmonary disease (COPD) who require treatment with a long- acting muscarinic receptor antagonist (LAMA) + long-acting beta2-receptor agonist (LABA) + inhaled corticosteroid (ICS). TRELEGY ELLIPTA should not be used for the initiation of COPD treatment. 4.2. Dose and method of administration Patients can be changed from their existing inhalers to TRELEGY ELLIPTA at the next dose. However it is important that patients do not take other LABA or LAMA or ICS while taking TRELEGY ELLIPTA. A stepwise approach to the management of COPD is recommended, including the cessation of smoking and a pulmonary rehabilitation program. -

1: Gastro-Intestinal System
1 1: GASTRO-INTESTINAL SYSTEM Antacids .......................................................... 1 Stimulant laxatives ...................................46 Compound alginate products .................. 3 Docuate sodium .......................................49 Simeticone ................................................... 4 Lactulose ....................................................50 Antimuscarinics .......................................... 5 Macrogols (polyethylene glycols) ..........51 Glycopyrronium .......................................13 Magnesium salts ........................................53 Hyoscine butylbromide ...........................16 Rectal products for constipation ..........55 Hyoscine hydrobromide .........................19 Products for haemorrhoids .................56 Propantheline ............................................21 Pancreatin ...................................................58 Orphenadrine ...........................................23 Prokinetics ..................................................24 Quick Clinical Guides: H2-receptor antagonists .......................27 Death rattle (noisy rattling breathing) 12 Proton pump inhibitors ........................30 Opioid-induced constipation .................42 Loperamide ................................................35 Bowel management in paraplegia Laxatives ......................................................38 and tetraplegia .....................................44 Ispaghula (Psyllium husk) ........................45 ANTACIDS Indications: -
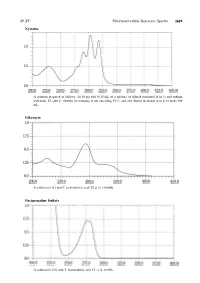
Nystatin Ofloxacin Orciprenaline Sulfate
JP XV Ultraviolet-visible Reference Spectra 1619 Nystatin A solution prepared as follows: To 10 mg add 50.25 mL of a mixture of diluted methanol (4 in 5) and sodium hydroxide TS (200:1), dissolve by warming at not exceeding 509C,andadddilutedmethanol(4in5)tomake500 mL. Ofloxacin Asolutionin0.1mol/LhydrochloricacidTS(1in150,000) Orciprenaline Sulfate A solution in 0.01 mol/L hydrochloric acid TS (1 in 10,000) 1620 Ultraviolet-visible Reference Spectra JP XV Oxazolam A solution in ethanol (95) (1 in 100,000) Oxethazaine A solution in ethanol (95) (1 in 2500) Oxybuprocaine Hydrochloride An aqueous solution (1 in 100,000) JP XV Ultraviolet-visible Reference Spectra 1621 Oxycodone Hydrochloride Hydrate An aqueous solution (1 in 10,000) Oxymetholone A solution prepared as follows: To 5 mL of a solution in methanol (1 in 5000) add 5 mL of sodium hydroxide-methanol TS and methanol to make 50 mL. Oxytetracycline Hydrochloride Asolutionin0.1mol/LhydrochloricacidTS(1in50,000) 1622 Ultraviolet-visible Reference Spectra JP XV Oxytocin An aqueous solution (1 in 2000) Penbutolol Sulfate A solution in methanol (1 in 10,000) Pentazocine A solution in 0.01 mol/L hydrochloric acid TS (1 in 10,000) JP XV Ultraviolet-visible Reference Spectra 1623 Peplomycin Sulfate Asolutionpreparedasfollows:To4mgadd5mL of copper (II) sulfate TS, and dissolve in water to make 100 mL. Perphenazine 1 Asolutionin0.1mol/LhydrochloricacidTS(1in200,000) Perphenazine 2 A solution obtained by adding 10 mL of water to 10 mL of the solution for Perphenazine 1 1624 Ultraviolet-visible