Mechanical Properties of Apple Skin Are Determined by Epidermis and Hypodermis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
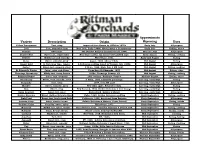
Variety Description Origin Approximate Ripening Uses
Approximate Variety Description Origin Ripening Uses Yellow Transparent Tart, crisp Imported from Russia by USDA in 1870s Early July All-purpose Lodi Tart, somewhat firm New York, Early 1900s. Montgomery x Transparent. Early July Baking, sauce Pristine Sweet-tart PRI (Purdue Rutgers Illinois) release, 1994. Mid-late July All-purpose Dandee Red Sweet-tart, semi-tender New Ohio variety. An improved PaulaRed type. Early August Eating, cooking Redfree Mildly tart and crunchy PRI release, 1981. Early-mid August Eating Sansa Sweet, crunchy, juicy Japan, 1988. Akane x Gala. Mid August Eating Ginger Gold G. Delicious type, tangier G Delicious seedling found in Virginia, late 1960s. Mid August All-purpose Zestar! Sweet-tart, crunchy, juicy U Minn, 1999. State Fair x MN 1691. Mid August Eating, cooking St Edmund's Pippin Juicy, crisp, rich flavor From Bury St Edmunds, 1870. Mid August Eating, cider Chenango Strawberry Mildly tart, berry flavors 1850s, Chenango County, NY Mid August Eating, cooking Summer Rambo Juicy, tart, aromatic 16th century, Rambure, France. Mid-late August Eating, sauce Honeycrisp Sweet, very crunchy, juicy U Minn, 1991. Unknown parentage. Late Aug.-early Sept. Eating Burgundy Tart, crisp 1974, from NY state Late Aug.-early Sept. All-purpose Blondee Sweet, crunchy, juicy New Ohio apple. Related to Gala. Late Aug.-early Sept. Eating Gala Sweet, crisp New Zealand, 1934. Golden Delicious x Cox Orange. Late Aug.-early Sept. Eating Swiss Gourmet Sweet-tart, juicy Switzerland. Golden x Idared. Late Aug.-early Sept. All-purpose Golden Supreme Sweet, Golden Delcious type Idaho, 1960. Golden Delicious seedling Early September Eating, cooking Pink Pearl Sweet-tart, bright pink flesh California, 1944, developed from Surprise Early September All-purpose Autumn Crisp Juicy, slow to brown Golden Delicious x Monroe. -

Germplasm Sets and Standardized Phenotyping Protocols for Fruit Quality Traits in Rosbreed
Germplasm Sets and Standardized Phenotyping Protocols for Fruit Quality Traits in RosBREED Jim Luby, Breeding Team Leader Outline of Presentation RosBREED Demonstration Breeding Programs Standardized Phenotyping Protocols Reference Germplasm Sets SNP Detection Panels Crop Reference Set Breeding Pedigree Set RosBREED Demonstration Breeding Programs Clemson U WSU Texas A&M UC Davis U Minn U Arkansas Rosaceae Cornell U WSU MSU MSU Phenotyping Affiliates USDA-ARS Driscolls Corvallis Univ of Florida UNH Standardized Phenotyping Protocols Traits and Standardized Phenotyping Protocols • Identify critical fruit quality traits and other important traits • Develop standardized phenotyping protocols to enable data pooling across locations/institutions • Protocols available at www.RosBREED.org Apple Standardized Phenotyping Firmness, Crispness – Instrumental, Sensory Sweetness, Acidity – Intstrumental, Sensory Color, Appearance, Juiciness, Aroma – Sensory At harvest Cracking, Russet, Sunburn Storage 10w+7d Storage 20w+7d Maturity Fruit size 5 fruit (reps) per evaluation Postharvest disorders Harvest date, Crop, Dropping RosBREED Apple Phenotyping Locations Wenatchee, WA St Paul, MN Geneva, NY • One location for all evaluations would reduce variation among instruments and evaluators • Local evaluations more sustainable and relevant for future efforts at each institution • Conduct standardized phenotyping of Germplasm Sets at respective sites over multiple (2-3) seasons • Collate data in PBA format, conduct quality control, archive Reference -

National Apple Orchard Census 2012
National Apple Orchard Census 2012 1 Table of Contents Introduction Page 3 Executive Summary Page 4 Section 1 Orchard Area, Sales, Growers and Markets Page 6 Section 2 Varieties and Orchard Age Page 15 Section 3 Employment, Facilities, Business Development and Producer Page 21 Profile Appendix Tables Table 1: Apple Production Area (hectares) by Year of Planting and Apple Type in Ireland in 2012 Table 2: Number of Apple Trees by Year of Planting and Apple Type in Ireland in 2012 Table 3: Number of Growers of Each Apple Type by County in Ireland in 2012 Table 4: Sales of 2011 Harvest by Market by County Table 5: Production Area, Number of Growers and Sales for Most Commonly Grown Apple Varieties in Ireland in 2011/12 Table 6: Average (median) Prices per Tonne by Market for 2011 harvest Table 7: Apple Production Area (hectares) in Ireland in 1980-2012 2 National Apple Orchard Census 2012 Introduction The National Apple Orchard Census 2012 was compiled from information provided by the 45 commercial apple growers operating in the Republic of Ireland in 2012. The census provides an important overview of apple production, and comparisons are made to previous censuses (2007, 2002 and 1997) where possible. The census surveyed all commercial orchards, defined as follows: - a production area of at least 0.2 hectares of apples, including cider apples - the fruit from the orchard is sold every year - the orchard is sprayed against pests/diseases every year, or maintained to organic standards Within the 45 growers, there are two organic growers. Production areas are based on areas planted up to March 2012. -

INF03 Reduce Lists of Apple Varieites
ECE/TRADE/C/WP.7/GE.1/2009/INF.3 Specialized Section on Standardization of Fresh Fruit and Vegetables Fifty-fifth session Geneva, 4 - 8 May 2009 Items 4(a) of the provisional agenda REVISION OF UNECE STANDARDS Proposals on the list of apple varieties This note has been put together by the secretariat following the decision taken by the Specialized Section at its fifty-fourth session to collect information from countries on varieties that are important in international trade. Replies have been received from the following countries: Canada, Czech Republic, Finland, France, Germany, Italy, Netherlands, New Zealand, Poland, Slovakia, South Africa, Sweden, Switzerland and the USA. This note also includes the documents compiled for the same purpose and submitted to the fifty-second session of the Specialized Section. I. Documents submitted to the 52nd session of the Specialized Section A. UNECE Standard for Apples – List of Varieties At the last meeting the 51 st session of the Specialized Section GE.1 the delegation of the United Kingdom offered to coordinate efforts to simplify the list of apple varieties. The aim was to see what the result would be if we only include the most important varieties that are produced and traded. The list is designed to help distinguish apple varieties by colour groups, size and russeting it is not exhaustive, non-listed varieties can still be marketed. The idea should not be to list every variety grown in every country. The UK asked for views on what were considered to be the most important top thirty varieties. Eight countries sent their views, Italy, Spain, the Netherlands, USA, Slovakia, Germany Finland and the Czech Republic. -

Pipfruit in Holland (Slideshow)
Fruitconsult - www.fruitconsult.com Fruitconsult - www.fruitconsult.com • Dutch consulting company • Working area • Based in Randwijk, – Holland Netherlands – Belgium • 7 advisors – Germany – pip- and stone fruits – Scandinavia – different specialists – Poland • nutrition/irrigation – Tsjechien • plant management – Hungary • plant protection – Italy – United Kingdom Pip fruit industry in Holland - mainly situated on river or sea sedi- Pip fruit industry in Holland ments (high pH -clays soils) - 700-900 mm rainfall/yr - 1500 professional growers - moderate climate # family run businesses - 5 main area’s - 17.000 ha pip fruit - Betuwe (Tiel/Geldermalsen) - Average farm size 11 ha - Utrecht - Zeeland (Goes) - number of growers decreases - Noord Holland (Hoorn) - average farm size increases quite fast - Zuid Limburg (Maastricht) Average production level: Apple industry in Holland 1990: 34.000kg/ha 1990: 20.000 ha 2009:45.000 kg/ha 2010: 8.500 ha Upper 10%: 60-65.000 kg/ha 1 Development in apple varieties in Nl Club varieties – Elstar 40% of production – Jonagold30% – Golden delicious6% – Boskoop6% – Cox‘s, Delcorf etc8% – New varieties • Kanzi400 ha • Rubens150 ha • Junami400 ha • Wellant100 ha • Pinova - Evelina • Topaz - Evita • Maribelle Club varieties Club varieties – only superior quality is sold • Strong tendency to club varieties – apples with minor deficiencies: <45% colour, light hail damage etc • 2009 -> 1000 ha = 12-13% -> industry • In 2009 10.000 tonnes premium • High and early production in order to keep the quality of different -

Grafting Fruit Trees
Grafting Fruit Trees By Glossary of Grafting Terms Scion Rootstock • Grafting-the process of inserting a part of one plant into or on another in a way that they will unite and continue growth as a single unit. What the Scion Brings to the Union • Scion—A piece of last year's growth with two or three buds (genetic matilterial for vegetative—asexual propagation); the part inserted on the understock or what we will call rootstock. Under stock (rootstock) 1 Why is it necessary to vegetatively propagate most tree fruit and nut cultivars by grafting (or budding)? ● Vegetative (Asexual) propagation maintains the genetic identity of the offspring Scion: A detached shoot or twig containing buds from a woody plant, used in grafting. Alternate definition: A descendant; an heir; as, a scion of a royal stock. ● Trees are grafted (or budded) because they are often difficult to root or ● they benefit from characteristics of the rootstock variety. Sexual propagation…(its all in the genes) Cultivar “A” Cultivar “B” .. allows for genetic mixing and recombination that requires a number of steps for diploid parents. ..They must first form haploid gametocytes, and that means their diploid chromosomes must partition themselves into two sets. ..This partitioning can be called genetic segregation. Only a few are selected Dog Either or It takes a tremendous amount of time, effort, and screening process to determine whether Winner one of out of thousands or more resultant prodigies is discarded (a dog) or of commercial value (a winner). 2 Honeycrisp Dog or winner • Honeycrisp (Malus domestica 'Honeycrisp') is an apple cultivar developed at the Minnesota Agricultural Experiment Station's Horticultural Research Center. -

Founding Clones, Inbreeding, Coancestry, and Status Number of Modern Apple Cultivars
J. AMER. SOC. HORT. SCI. 121(5):773–782. 1996. Founding Clones, Inbreeding, Coancestry, and Status Number of Modern Apple Cultivars Dominique A.M. Noiton The Horticulture and Food Research Institute of New Zealand Ltd, Havelock North Research Center, Havelock North, New Zealand Peter A. Alspach The Horticulture and Food Research Institute of New Zealand Ltd, Riwaka Research Center, Motueka, New Zealand Additional index words. breeding, genetic diversity, Malus ×domestica Abstract. Pedigrees of apple (Malus ×domestica Borkh.) cultivars were used to study worldwide genetic diversity among clones used in modern apple breeding. The most frequent founding clones were ‘Cox’s Orange Pippin’, ‘Golden Delicious’, ‘Red Delicious’, ‘Jonathan’, and ‘McIntosh’. Coefficients of coancestry between 50 mainstream cultivars and these clones averaged 0.03, 0.12, 0.07, 0.06, and 0.02, respectively, but they were frequently as high as 0.25 with certain pairings. Among a group of 27 cultivars carrying the Vf gene for scab resistance, coefficients of coancestry with the five founding clones were of the same order. Although few of the cultivars sampled were substantially inbred, inbreeding could reach serious levels in their future offspring if current breeding practices are continued. The status effective number was 8 for the mainstream group and 7 for the Vf-carrier clones. This indicates clearly that apple breeders are operating with a population of greatly reduced genetic diversity. Careful consideration of pedigrees and increased size of the genetic base are needed in future apple breeding strategies. The domestic apple (Malus ×domestica), one of the world’s floribunda 821 x ‘Rome Beauty’. -

Apple Pollination Groups
Flowering times of apples RHS Pollination Groups To ensure good pollination and therefore a good crop, it is essential to grow two or more different cultivars from the same Flowering Group or adjacent Flowering Groups. Some cultivars are triploid – they have sterile pollen and need two other cultivars for good pollination; therefore, always grow at least two other non- triploid cultivars with each one. Key AGM = RHS Award of Garden Merit * Incompatible with each other ** Incompatible with each other *** ‘Golden Delicious’ may be ineffective on ‘Crispin’ (syn. ‘Mutsu’) Flowering Group 1 Very early; pollinated by groups 1 & 2 ‘Gravenstein’ (triploid) ‘Lord Suffield’ ‘Manks Codlin’ ‘Red Astrachan’ ‘Stark Earliest’ (syn. ‘Scarlet Pimpernel’) ‘Vista Bella’ Flowering Group 2 Pollinated by groups 1,2 & 3 ‘Adams's Pearmain’ ‘Alkmene’ AGM (syn. ‘Early Windsor’) ‘Baker's Delicious’ ‘Beauty of Bath’ (partial tip bearer) ‘Beauty of Blackmoor’ ‘Ben's Red’ ‘Bismarck’ ‘Bolero’ (syn. ‘Tuscan’) ‘Cheddar Cross’ ‘Christmas Pearmain’ ‘Devonshire Quarrenden’ ‘Egremont Russet’ AGM ‘George Cave’ (tip bearer) ‘George Neal’ AGM ‘Golden Spire’ ‘Idared’ AGM ‘Irish Peach’ (tip bearer) ‘Kerry Pippin’ ‘Keswick Codling’ ‘Laxton's Early Crimson’ ‘Lord Lambourne’ AGM (partial tip bearer) ‘Maidstone Favourite’ ‘Margil’ ‘Mclntosh’ ‘Red Melba’ ‘Merton Charm’ ‘Michaelmas Red’ ‘Norfolk Beauty’ ‘Owen Thomas’ ‘Reverend W. Wilks’ ‘Ribston Pippin’ AGM (triploid, partial tip bearer) ‘Ross Nonpareil’ ‘Saint Edmund's Pippin’ AGM (partial tip bearer) ‘Striped Beefing’ ‘Warner's King’ AGM (triploid) ‘Washington’ (triploid) ‘White Transparent’ Flowering Group 3 Pollinated by groups 2, 3 & 4 ‘Acme’ ‘Alexander’ (syn. ‘Emperor Alexander’) ‘Allington Pippin’ ‘Arthur Turner’ AGM ‘Barnack Orange’ ‘Baumann's Reinette’ ‘Belle de Boskoop’ AGM (triploid) ‘Belle de Pontoise’ ‘Blenheim Orange’ AGM (triploid, partial tip bearer) ‘Bountiful’ ‘Bowden's Seedling’ ‘Bramley's Seedling’ AGM (triploid, partial tip bearer) ‘Brownlees Russett’ ‘Charles Ross’ AGM ‘Cox's Orange Pippin’ */** ‘Crispin’ (syn. -

Fructose in Different Apple Varieties
Copyright! Reproduction and dissemination – also partial – applicable to all media only with written permission of Umschau Zeitschriftenverlag GmbH, Wiesbaden. Science & Research | Original Contribution Peer-reviewed | Manuscript received: March 23, 2017 | Revision accepted: November 28, 2017 Fructose in different apple varieties Implications for apple consumption in persons affected by fructose intolerance Katharina Hermann, Ursula Bordewick-Dell Fructose malabsorption is often er- Abstract roneously referred to in the media as Fructose malabsorption (FM) and hereditary fructose intolerance (HFI) are path- fructose intolerance, which means ological diseases that should be clearly distinguished from one another, but for that it can be easily confused with both of which fructose is a relevant substrate. The treatment for both diseases the metabolic disease hereditary consists of fructose restriction, but with a completely different focus for each. In fructose intolerance [2]. The diffe- the case of HFI, consumption of fructose must be avoided completely, but in the rent forms of fructose intolerance case of FM, a desensitization strategy can be adopted. This strategy exploits the should be clearly distinguished mechanism of glucose-induced fructose transport. Thus, for FM (unlike for HFI) from one another [3]. • Table 1 pro- it is not necessary to abstain from consuming foods containing fructose comple- vides a comparison of HFI and FM tely. with regard to their pathomecha- Apples have a high fructose content, and also have an unfavorable fructose/glu- nisms, symptoms, diagnosis, and cose ratio of > 1, which means that at first glance, they appear unsuitable for treatment. In both of these diseases, patients with FM. treatment is based on nutritional therapy with fructose restriction Our investigations have shown that depending on the variety, the fructose con- playing a key role, but the focus of tent of apples can vary considerably. -

Apple Varieties at Lathcoats Farm
Apple Varieties at Lathcoats Farm All the varieties listed below are available, ready picked, in season in our farm shop. All the apples are grown in our orchards here in Galleywood. VARIETY DATE PLACE OF ORIGIN ON SALE SEASON DISCOVERY 1949 ESSEX AUG SHORT FESTIVAL 1950s FRANCE AUG SHORT QUEEN 1858 BILLERICAY AUG MEDIUM TYDEMAN’S EARLY 1929 KENT AUG SHORT LAXTON’S FORTUNE 1904 BEDFORD SEP SHORT WORCESTER PEARMAIN 1894 WORCESTER SEP SHORT JAMES GRIEVE 1893 SCOTLAND SEP SHORT ALKMENE 1930 GERMANY SEP SHORT BRAMLEY 1809 NOTTS SEP LONG LORD LAMBOURNE 1907 BEDFORD SEP MEDIUM CHELMSFORD WONDER 1870 CHELMSFORD SEP MEDIUM ST EDMUND’S PIPPIN 1875 SUFFOLK SEP SHORT RED ELSTAR 1972 HOLLAND SEP MEDIUM ADAMS PEARMAIN 1826 HEREFORD OCT MEDIUM CORNISH GILLIFLOWER C1800 CORNWALL OCT MEDIUM HONEYCRISP C1960 MINNESOTA OCT MEDIUM TEMPTATION 1990 FRANCE OCT MEDIUM TOPAZ LATE C20 CZECH REPUBLIC OCT MEDIUM MERIDAN 2000 KENT OCT MEDIUM PINOVA 1986 GERMAY OCT MEDIUM CRISPIN 1930 JAPAN OCT MEDIUM COX’S ORANGE PIPPIN 1825 BUCKS OCT LONG Please Turn Over for more varieties Page | 1 Apple Varieties at Lathcoats Farm All the varieties listed below are available, ready picked, in season in our farm shop. All the apples are grown in our orchards here in Galleywood. VARIETY DATE PLACE OF ORIGIN ON SALE SEASON EGREMONT RUSSET 1872 UK OCT LONG BLENHEIM ORANGE 1740 OXON OCT MEDIUM SPARTAN 1926 CANADA OCT MEDIUM ASHMEAD’S KERNEL 1700 GLOUCESTER OCT MEDIUM GALA 1934 NEW ZEALAND SEP LONG RIBSTON PIPPIN 1707 YORKS OCT MEDIUM KIDDS ORANGE 1924 NEW ZEALAND OCT MEDIUM JONAGORED 1943 USA OCT LONG LAXTON’S SUPERB 1897 BEDFORD OCT SHORT CHIVERS DELIGHT 1936 CAMBS OCT MEDIUM FALSTAFF 1966 KENT OCT MEDIUM WINTER GEM 1985 KENT OCT SHORT EDEN 1971 QUEBEC OCT LIMITED CHRISTMAS PIPPIN 2011 SOMERSET OCT LIMITED LITTLE PAX 2014 ISLE OF WIGHT OCT LIMITED BRAEBURN 1952 NEW ZEALAND NOV LONG D’ARCY SPICE 1785 ESSEX NOV MEDIUM LATHCOATS RED JOYCE’S CHOICE Page | 2 . -

Successful Biological Orcharding
Successful Biological Orcharding Applying nature's tenets to grow outrageously good fruit Fascinating biological connections make for a healthy orchard ecosystem. All insect pests and fruit tree disease – whether fungal or bacterial – have launching points and particular timing. Healthy trees address these challenges first and foremost from within. Growers utilizing an ongoing investment in soil nutrition and biodiversity set the stage for gentler organic sprays to grow a successful fruit crop. The challenges you face at your locale will become far more manageable as you build a holistic system that keeps trees and berry plantings healthy from the get-go. COMMUNITY ORCHARD FOCUS: We’ll wrap up this day with important marketing perspective for selling the good fruit. 1 diversified farm photo The Right Size Orchard • Economics of more and more acreage • Peak labor times call for ingenuity • Farm as organism • Resilience factors • Community markets • Having fun! 2 Hoch Family Orchard learning curve complexity 3 Healthy Plant Metabolism • Sunshine launches plant The Making of metabolism. • Nitrogen combines with a Healthy Plant plant sugars to create proteins. • Fat energy drives the cuticle defense • Resistance metabolites provide “immune function” against disease and higher order insects 4 photosynthesis Photosynthesis Efficiency • Mn, Cl, and B are activators of enzymes. • Cu, Fe, Zn, and Mo are components of enzymes. • Micronutrients play a key role in protein synthesis as well. 5 The form of Protein Synthesis nitrogen uptake by tree roots plays a significant role in the tree’s innate ability to resist disease. The Right Nitrogen Susceptibility to disease goes rocketing up whenever an orchard tree takes in nutrition in a form that undermines immune function. -

Molecular Genetic Identification of Apple Cultivars Based On
Erwerbs-Obstbau (2020) 62:117–154 https://doi.org/10.1007/s10341-020-00483-0 ORIGINAL ARTICLE Molecular Genetic Identification of Apple Cultivars Based on Microsatellite DNA Analysis. I. The Database of 600 Validated Profiles Sanja Baric1,2 · Alberto Storti1,2 ·MelanieHofer1 ·WalterGuerra1 · Josef Dalla Via3 Received: 12 March 2020 / Accepted: 24 March 2020 / Published online: 28 April 2020 © The Author(s) 2020 Abstract Apple (Malus× domestica Borkh.) is the most widely grown permanent fruit crop of temperate climates. Although com- mercial apple growing is based on a small number of globally spread cultivars, its diversity is much larger and there are estimates about the existence of more than 10,000 documented varieties. The varietal diversity can be described and determined based on phenotypic characters of the external and internal traits of fruit, which, however, can be modulated by environmental factors. Consequently, molecular methods have become an important alternative means for the char- acterisation of apple cultivar diversity. In order to use multilocus microsatellite data for determination of unidentified or misidentified apple varieties, a database with molecular genetic fingerprints of well-determined reference cultivars needs to be available. The objective of the present work was to establish such a database that could be applied for the molecular genetic determination of a large number of historic and modern, diploid and triploid apple cultivars. Based on the analysis of more than 1600 accessions of apple trees sampled in 37 public and private cultivar collections in different European countries at 14 variable microsatellite loci, a database with 600 molecular genetic profiles was finally obtained.