Studies Related on the Thesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

IHS National Pharmacy & Therapeutics Committee National
IHS National Pharmacy & Therapeutics Committee National Core Formulary; Last Updated: 09/23/2021 **Note: Medications in GREY indicate removed items.** Generic Medication Name Pharmacological Category (up-to-date) Formulary Brief (if Notes / Similar NCF Active? available) Miscellaneous Medications Acetaminophen Analgesic, Miscellaneous Yes Albuterol nebulized solution Beta2 Agonist Yes Albuterol, metered dose inhaler Beta2 Agonist NPTC Meeting Update *Any product* Yes (MDI) (Nov 2017) Alendronate Bisphosphonate Derivative Osteoporosis (2016) Yes Allopurinol Antigout Agent; Xanthine Oxidase Inhibitor Gout (2016) Yes Alogliptin Antidiabetic Agent, Dipeptidyl Peptidase 4 (DPP-4) Inhibitor DPP-IV Inhibitors (2019) Yes Anastrozole Antineoplastic Agent, Aromatase Inhibitor Yes Aspirin Antiplatelet Agent; Nonsteroidal Anti-Inflammatory Drug; Salicylate Yes Azithromycin Antibiotic, Macrolide STIs - PART 1 (2021) Yes Calcium Electrolyte supplement *Any formulation* Yes Carbidopa-Levodopa (immediate Anti-Parkinson Agent; Decarboxylase Inhibitor-Dopamine Precursor Parkinson's Disease Yes release) (2019) Clindamycin, topical ===REMOVED from NCF=== (See Benzoyl Peroxide AND Removed January No Clindamycin, topical combination) 2020 Corticosteroid, intranasal Intranasal Corticosteroid *Any product* Yes Cyanocobalamin (Vitamin B12), Vitamin, Water Soluble Hematologic Supplements Yes oral (2016) Printed on 09/25/2021 Page 1 of 18 National Core Formulary; Last Updated: 09/23/2021 Generic Medication Name Pharmacological Category (up-to-date) Formulary Brief -

Drugs Affectin the Autonomic Nervous System
Fundamentals of Medical Pharmacology Paterson Public Schools Written by Néstor Collazo, Ph.D. Jonathan Hodges, M.D. Tatiana Mikhaelovsky, M.D. for Health and Related Professions (H.A.R.P.) Academy March 2007 Course Description This fourth year course is designed to give students in the Health and Related Professions (H.A.R.P.) Academy a general and coherent explanation of the science of pharmacology in terms of its basic concepts and principles. Students will learn the properties and interactions between chemical agents (drugs) and living organisms for the rational and safe use of drugs in the control, prevention, and therapy of human disease. The emphasis will be on the fundamental concepts as they apply to the actions of most prototype drugs. In order to exemplify important underlying principles, many of the agents in current use will be singled out for fuller discussion. The course will include the following topics: ¾ The History of Pharmacology ¾ Terminology Used in Pharmacology ¾ Drug Action on Living Organisms ¾ Principles of Pharmacokinetics ¾ Dose-Response Relationships ¾ Time-Response Relationships ¾ Human Variability: Factors that will modify effects of drugs on individuals ¾ Effects of Drugs Attributable to Varying Modes of Administration ¾ Drug Toxicity ¾ Pharmacologic Aspects of Drug Abuse and Drug Dependence Pre-requisites Students must have completed successfully the following courses: Biology, Chemistry, Anatomy and Physiology, Algebra I and II Credits: 5 credits Basic Principles of Drug Action Introduction to Pharmacology a. Basic Mechanisms of Drug Actions b. Dose-response relationships c. Drug absorption d. Biotransformation of Drugs e. Pharmacokinetics f. Factors Affecting Drug Distribution g. Drug Allergy and Pharmacogenetics h. -

Rhode Island Chapter Abstracts
Rhode Island Chapter Abstracts April 1, 2015 Abdin, Ahmad Last Name: Abdin First Author: Resident First Name: Ahmad Category: Clinical Vignette PG Year: PGY-1 or MS Year: ACP Number: 2888549 Medical School or Residency Program: Warren Alpert Medical School of Brown University Hospital Affiliation: Memorial Hospital of Rhode Island, Providence VA Medical Center Additional Authors: Ahmad Abdin, MD, Mohammed Salhab, MD, Amos Charles, MD, Mazen Al-Qadi, MD Abstract Title: Amiodarone-Induced Cerebellar Dysfunction Abstract Text: Introduction: Amiodarone is a class III antiarrhythmic agent that is widely used to treat ventricular and supraventricular tachycardias. Several side-effects of the drug have been recognized including thyroid dysfunction, photosensitivity, hepatotoxicity, parenchymal lung disease, corneal deposits, and peripheral neuropathy. Cerebellar dysfunction is rarely seen in patients receiving amiodarone. We are reporting a rare case of amiodarone-induced cerebellar dysfunction that resolved completely upon discontinuation of the drug. Case Presentation: A 73-year-old man with a past medical history significant for paroxysmal atrial fibrillation, coronary artery disease, diabetes and hypertension who presented with worsening lower extremity weakness and unsteady gait with recurrent falls for the last 6 months. Two days prior to admission, his symptoms got worse and caused him to seek medical attention. Two years ago he was started and maintained on amiodarone 200 mg daily for rhythm control. On physical examination, vital signs were normal. A wide-based unsteady gait was noted. He had dysmetria bilaterally on finger-to-nose and heel-to-shin testing. The rapid alternating movements of the hands were irregular. The remainder of the general and neurologic examinations were unremarkable. -

Class I and Class III Antiarrhythmic Agents: Mechanisms of Action and the Problem of Proarrhythmic Activity
Class I and Class III Antiarrhythmic Agents: Mechanisms of Action and the Problem of Proarrhythmic Activity Vidal Essebag* * To whom correspondence should be addressed: Faculty of Medicine, McGill University, Montreal, QC, Canada H3G 1Y6 INTRODUCTION Sudden cardiac death is a leading cause of mortality in industrialized nations, accounting for 50% of all cardiovascular deaths (1). Despite a decline in the past 20 years, sudden cardiac death accounts for 300,000 fatalities per year in the United States. Approximately 60% of these deaths occur in the absence of previously diagnosed heart disease (1). Nevertheless, in most instances of sudden cardiac death, the underlying pathophysiology of atherosclerotic cardiac disease with the superimposition of transient ischemic events makes the heart more susceptible to the onset and maintenance of lethal arrhythmias (1). Approximately 50% of post-myocardial infarction (MI) fatalities are sudden cardiac deaths resulting mostly from ventricular tachycardia (VT) or ventricular fibrillation (VF). It is believed that these arrhythmias arise from mechanical dysfunction and ischemic events interacting within a disordered electrophysiologic milieu (2). This has prompted the active search for safe and effective treatment modalities and their ultimate evaluation in clinical trials. Currently, beta blockers (class II agents) are recommended for post-MI patients with frequent premature ventricular contractions (PVCs), whereas calcium antagonists (class IV agents) are not helpful, and class I agents are actually harmful (2). The benefit of class III drugs, particularly amiodarone, is presently being evaluated in large clinical trials. Therapeutic options for patients with sustained VT or VF occurring late after MI include pharmacologic antiarrhythmic therapy, transcatheter or surgical ablative therapy (for VT), and implantable cardioverter-defibrillators. -

TENORMIN® (Atenolol) Tablets
® TENORMIN (atenolol) Tablets DESCRIPTION ® TENORMIN (atenolol), a synthetic, beta1-selective (cardioselective) adrenoreceptor blocking agent, may be chemically described as benzeneacetamide, 4 -[2'-hydroxy-3'-[(1- methylethyl) amino] propoxy]-. The molecular and structural formulas are: C14H22N2O3 Atenolol (free base) has a molecular weight of 266. It is a relatively polar hydrophilic compound with a water solubility of 26.5 mg/mL at 37°C and a log partition coefficient (octanol/water) of 0.23. It is freely soluble in 1N HCl (300 mg/mL at 25°C) and less soluble in chloroform (3 mg/mL at 25°C). TENORMIN is available as 25, 50 and 100 mg tablets for oral administration. Inactive Ingredients: Magnesium stearate, microcrystalline cellulose, povidone, sodium starch glycolate. CLINICAL PHARMACOLOGY TENORMIN is a beta1-selective (cardioselective) beta-adrenergic receptor blocking agent without membrane stabilizing or intrinsic sympathomimetic (partial agonist) activities. This preferential effect is not absolute, however, and at higher doses, TENORMIN inhibits beta2-adrenoreceptors, chiefly located in the bronchial and vascular musculature. Pharmacokinetics and Metabolism In man, absorption of an oral dose is rapid and consistent but incomplete. Approximately 50% of an oral dose is absorbed from the gastrointestinal tract, the remainder being excreted unchanged in the feces. Peak blood levels are reached between two (2) and four (4) hours after ingestion. Unlike propranolol or metoprolol, but like nadolol, TENORMIN undergoes little or no metabolism by the liver, and the absorbed portion is eliminated primarily by renal excretion. Over 85% of an intravenous dose is excreted in urine within 24 hours compared with approximately 50% for an oral dose. -

Cordarone (Amiodarone Hydrochloride)
Cordarone® (amiodarone HCl) TABLETS Rx only DESCRIPTION Cordarone (amiodarone HCl) is a member of a class of antiarrhythmic drugs with predominantly Class III (Vaughan Williams’ classification) effects, available for oral administration as pink, scored tablets containing 200 mg of amiodarone hydrochloride. The inactive ingredients present are colloidal silicon dioxide, lactose, magnesium stearate, povidone, starch, and FD&C Red 40. Cordarone is a benzofuran derivative: 2-butyl-3 benzofuranyl 4-[2-(diethylamino)-ethoxy]-3,5-diiodophenyl ketone hydrochloride. The structural formula is as follows: Amiodarone HCl is a white to cream-colored crystalline powder. It is slightly soluble in water, soluble in alcohol, and freely soluble in chloroform. It contains 37.3% iodine by weight. CLINICAL PHARMACOLOGY Electrophysiology/Mechanisms of Action In animals, Cordarone is effective in the prevention or suppression of experimentally induced arrhythmias. The antiarrhythmic effect of Cordarone may be due to at least two major properties: 1. a prolongation of the myocardial cell-action potential duration and refractory period and 2. noncompetitive α- and β-adrenergic inhibition. Cordarone prolongs the duration of the action potential of all cardiac fibers while causing minimal reduction of dV/dt (maximal upstroke velocity of the action potential). The refractory period is prolonged in all cardiac tissues. Cordarone increases the cardiac refractory period without influencing resting membrane potential, except in automatic cells where the slope of the prepotential is reduced, generally reducing automaticity. These electrophysiologic effects are reflected in a decreased sinus rate of 15 to 20%, increased PR and QT intervals of about 10%, the development of U-waves, and changes in T-wave contour. -
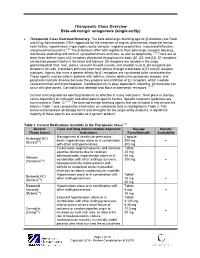
Therapeutic Class Overview Beta-Adrenergic Antagonists (Single-Entity)
Therapeutic Class Overview Beta-adrenergic antagonists (single-entity) · Therapeutic Class Overview/Summary: The beta-adrenergic blocking agents (β-blockers) are Food and Drug Administration (FDA)-approved for the treatment of angina, arrhythmias, essential tremor, heart failure, hypertension, hypertrophic aortic stenosis, migraine prophylaxis, myocardial infarction, and pheochromocytoma.1-26 The β-blockers differ with regards to their adrenergic-receptor blocking, membrane stabilizing and intrinsic sympathomimetic activities, as well as lipophilicity.1-26 There are at least three distinct types of β receptors distributed throughout the body (β1, β2, and β3). β1-receptors are located predominantly in the heart and kidneys. β2-receptors are located in the lungs, gastrointestinal tract, liver, uterus, vascular smooth muscle, and skeletal muscle. β3-receptors are located in fat cells. β-blockers primarily exert their effects through a blockade of β1 and β2 receptor subtypes. Agents that have a greater affinity for β1 receptors are considered to be cardioselective. These agents may be safer in patients with asthma, chronic obstructive pulmonary disease, and peripheral vascular disease because they produce less inhibition of β2 receptors, which mediate vasoconstriction and bronchospasm. Cardioselectivity is dose dependent; therefore, β2 blockade can occur at higher doses. Carvedilol and labetalol also block α-adrenergic receptors. 27-28 Current clinical guidelines identify β-blockers as effective in many indications. Their place in therapy varies depending on indication and other patient specific factors. Specific treatment guidelines are summarized in Table 12.29-61 The beta-adrenergic blocking agents that are included in this review are listed in Table 1 and comparative information on cardioselectivity is highlighted in Table 2. -

2005 Changes to the American Heart Association Guidelines For
N E WS N E WS Pharmacotherapy Update LETTER Pharmacotherapyfrom the DepartmentUpdate of Pharmacy LETTER fromVolume the IX,Department No. II March/April of Pharmacy 2006 Mandy C. Leonard, Pharm.D., BCPS Assistant Director, Drug Information Service 2005 Changes to the American Heart Association Editor Guidelines for Cardiopulmonary Resuscitation Meghan K. Lehmann, Pharm.D., BCPS by Heather Bockheim, Pharm.D. Drug Information Specialist Editor The concept of resuscitation was first documented in 1740, when the Paris Academy of Sciences recommended mouth-to-mouth resuscitation for drowning Dana L. Travis, R.Ph. victims.1 Almost 200 years later, in 1903, Dr. George W. Crile, co-founder of Drug Information Pharmacist Editor the Cleveland Clinic, reported the first successful use of external chest compres- sions in human resuscitation.1 In 1957, the United States military adopted the David A. White, B.S., R.Ph. use of mouth-to-mouth resuscitation to aid in the revival of unresponsive Restricted Drug Pharmacist patients.1 Finally, in 1960, closed chest cardiopulmonary resuscitation (CPR) Associate Editor was developed. Shortly thereafter, the American Heart Association (AHA) Marcia J. Wyman, Pharm.D. began developing training programs for both physicians and the public (See 1 Drug Information Pharmacist Figure 1). Associate Editor In the United States, sudden cardiac arrest (SCA) accounts for 250,000 out-of- Amy T. Sekel, Pharm.D. 2 Drug Information Pharmacist hospital deaths each year. Despite enormous labors to encourage public aware- AssociateIn this IssueEditor ness and education, cardiopulmonary arrest remains a significant public health challenge worldwide. The survival rate for out-of-hospital SCA remains dismal David Kvancz, M.S., R.Ph., FASHP with 94% of victims dying prior to hospital arrival worldwide.2 Chief Pharmacy Officer Morton Goldman, Pharm.D., BCPS The revised AHA guidelines for CPR and emergency cardiovascular care 3 Director, Pharmacotherapy Services (ECC) were updated and published in December 2005. -

Hydroxychloroquine (All Populations Monograph)
3/17/2020 Clinical Pharmacology powered by ClinicalKey Hydroxychloroquine (All Populations Monograph) Indications/Dosage expand all | collapse all Labeled malaria rheumatoid arthritis malaria prophylaxis systemic lupus erythematosus (SLE) Off-Label, Recommended coronavirus disease 2019 (COVID-19) † severe acute respiratory syndrome coronavirus lupus nephritis † 2 (SARS-CoV-2) infection † polymorphous light eruption † † Off-label indication Per the manufacturer, this drug has been shown to be active against most strains of the following microorganisms either in vitro and/or in clinical infections: Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, Plasmodium vivax. NOTE: The safety and effectiveness in treating clinical infections due to organisms with in vitro data only have not been established in adequate and well-controlled clinical trials. This drug may also have activity against the following microorganisms: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). NOTE: Some organisms may not have been adequately studied during clinical trials; therefore, exclusion from this list does not necessarily negate the drug’s activity against the organism. For the treatment of malaria due to susceptible strains of P. falciparum, P. knowlesi†, P. malariae, P. ovale, and P. vivax https://www.clinicalkey.com/pharmacology/monograph/print?cpnum=300&type=0&printSections=monindi&printSections=monsup&printSections=mon… 1/87 3/17/2020 Clinical Pharmacology powered by ClinicalKey Oral dosage Adults 13 mg (10 mg base)/kg/dose [Max: 800 mg (620 mg base)/dose] PO, then 6.5 mg (5 mg base)/kg/dose [Max: 400 mg (310 mg base)/dose] PO at 6, 24, and 48 hours after the initial dose.[41806] [64059] For P. -

Table 1: Drug-Drug Interactions of Common Cardiac Drugs and Chemotherapeutic Agents*
Table 1: Drug-Drug Interactions of Common Cardiac Drugs and Chemotherapeutic Agents* Cardiac Drug(s) Enzyme/ Chemotherapy Effect of Drug- Suggested Oncologist Suggested Cardiologist Action Drug† Drug Management Management Interaction Beta-Blockers All beta- Additive Ceritinib Additive Avoid using the combination of ceritinib with beta- blockers clinical bradycardia blockers. If concomitant use is necessary and symptomatic effect bradycardia occurs, hold ceritinib, adjust or discontinue the beta-blocker, and upon recovery resume ceritinib at a reduced dose with frequent monitoring of heart rate.‡ Crizotinib Monitor blood pressure and heart rate regularly. Dose reduction or discontinuation of one of the agents may be necessary if clinically significant bradycardia occurs.‡ Carvedilol P-gp Afatinib ↑ Monitor for adverse Consider alternative agent if inhibition chemotherapy effects of afatinib. If possible. (moderate) drug not well-tolerated, concentration decrease afatinib daily dose by 10 mg. Doxorubicin Monitor for adverse Consider alternative agent if Nilotinib effects of possible. If carvedilol is used for Paclitaxel chemotherapy drug if prevention of anthracycline Pazopanib concomitant therapy is cardiotoxicity, individual risk vs. Vincristine necessary. benefit must be considered. If Vinblastine concomitant therapy is necessary and drug-drug interaction involves QT- prolonging chemotherapy drug, ensure appropriate electrocardiographic (ECG) and electrolyte monitoring. Carvedilol; CYP2D6 Imatinib ↑ beta-blocker Monitor blood pressure -

IHS National Pharmacy & Therapeutics Committee National
IHS National Pharmacy & Therapeutics Committee National Core Formulary; Last Updated: 09/23/2021 **Note: Medications in GREY indicate removed items.** Generic Pharmacological Category Formulary Brief (if Notes / Similar NCF Medications Active? Medication Name (up-to-date) available) Miscellaneous Acetaminophen Analgesic, Miscellaneous Yes Albuterol nebulized Beta2 Agonist Yes solution Alendronate Bisphosphonate Derivative Osteoporosis (2016) Yes Allopurinol Antigout Agent; Xanthine Oxidase Gout (2016) Yes Inhibitor Alogliptin Antidiabetic Agent, Dipeptidyl DPP-IV Inhibitors Yes Peptidase 4 (DPP-4) Inhibitor (2019) Amitriptyline Antidepressant, Tricyclic Antidepressants in Nortriptyline Yes Pain Mgmt (2014) Amlodipine Antihypertensive; Calcium Calcium Channel Diltiazem Yes Channel Blocker, Dihydropyridine Blockers (2014) Anastrozole Antineoplastic Agent, Aromatase Yes Inhibitor Apixaban Anticoagulant; Direct Oral Direct Oral Warfarin Yes Anticoagulant (DOAC); Factor Xa Anticoagulants (2017) Inhibitor Aspirin Antiplatelet Agent; Nonsteroidal Yes Anti-Inflammatory Drug; Salicylate Atenolol Antihypertensive; Beta-Blocker, Beta Blockers (2014) Metoprolol; Propranolol Yes Beta1 Selective Atomoxetine Norepinephrine Reuptake Inhibitor, ADHD (2020) Dextroamphetamine / Amphetamine (immediate release); Yes Selective Dextroamphetamine / Amphetamine (long-acting); Methylphenidate (immediate release); Methylphenidate (long-acting) Atorvastatin Antilipemic Agent, HMG-CoA Dyslipidemia Guideline Pravastatin; Rosuvastatin; Simvastatin Yes Reductase Inhibitor -

Cardiac Medications in a Nutshell
Cardiac Medications in a Nutshell Beta Blockers Atenolol Atenolol is a drug belonging to the group of β-blockers, a class of drugs used primarily in cardiovascular diseases. Introduced in 1976, atenolol was developed as a replacement for propranolol in the treatment of hypertension. The chemical works by slowing down the heart and reducing its workload. Atenolol (trade name Tenormin) can be used to treat cardiovascular diseases and conditions such as hypertension, coronary heart disease, arrhythmias, angina (chest pain) and to treat and reduce the risk of heart complications following myocardial infarction (heart attack) Carvedilol Carvedilol is a non-selective beta blocker indicated in the treatment of mild to moderate congestive heart failure (CHF). Carvedilol blocks the binding to those receptors, which both slows the heart rhythm and reduces the force of the heart's pumping. This lowers blood pressure and reduces heart failure. Sotalol Sotalol (trade names Betapace and Betapace AF) is a drug used in individuals with rhythm disturbances (cardiac arrhythmias) of the heart, and to treat hypertension in some individuals. It lengthens the QT interval. It also slows atrioventricular (AV) nodal conduction (beta-blocking effect). Sotalol is not selected for its beta-blocking ability, but rather for its Class III (potassium blocking) properties. Sotalol is used to treat ventricular tachycardias[2] as well as atrial fibrillation.[3] Betapace AF is specifically labeled for atrial fibrillation. It has also been suggested that it be used in the prevention of atrial fibrillation.[5] ACE Inhibitors Benazepril Benazepril, brand name Lotensin, is a medication used to treat high blood pressure (hypertension), congestive heart failure, and chronic renal failure.