Electroporation-Enhanced Gene Delivery in Mammary Tumors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

REVIEW Gene Therapy
Leukemia (2001) 15, 523–544 2001 Nature Publishing Group All rights reserved 0887-6924/01 $15.00 www.nature.com/leu REVIEW Gene therapy: principles and applications to hematopoietic cells VFI Van Tendeloo1,2, C Van Broeckhoven2 and ZN Berneman1 1Laboratory of Experimental Hematology, University of Antwerp (UIA), Antwerp University Hospital (UZA), Antwerp; and 2Laboratory of Molecular Genetics, University of Antwerp (UIA), Department of Molecular Genetics, Flanders Interuniversity Institute for Biotechnology (VIB), Antwerp, Belgium Ever since the development of technology allowing the transfer Recombinant viral vectors of new genes into eukaryotic cells, the hematopoietic system has been an obvious and desirable target for gene therapy. The last 10 years have witnessed an explosion of interest in this Biological gene transfer methods make use of modified DNA approach to treat human disease, both inherited and acquired, or RNA viruses to infect the cell, thereby introducing and with the initiation of multiple clinical protocols. All gene ther- expressing its genome which contains the gene of interest (= apy strategies have two essential technical requirements. ‘transduction’).1 The most commonly used viral vectors are These are: (1) the efficient introduction of the relevant genetic discussed below. In each case, recombinant viruses have had material into the target cell and (2) the expression of the trans- gene at therapeutic levels. Conceptual and technical hurdles the genes encoding essential replicative and/or packaging pro- involved with these requirements are still the objects of active teins replaced by the gene of interest. Advantages and disad- research. To date, the most widely used and best understood vantages of each recombinant viral vector are summarized in vectors for gene transfer in hematopoietic cells are derived Table 1. -

Gene Delivery & Expression
GENE DELIVERY & EXPRESSION GENE DELIVERY & EXPRESSION LENTIVIRUS, PIGGYBAC, MINICIRCLES, AND MORE SYSTEMBIO.COM SYSTEMS FOR ANY APPLICATION: LENTIVIRAL TOOLS AND MORE In today’s busy labs, getting experiments to work right the first time is critical—not only do you want results you can trust, you want them fast. Which is why SBI has spent over a decade developing a range of exceptionally high-performing products for gene delivery and expression. From our popular high-titer lentivirus production tools to the latest in AAV-based gene delivery systems and a range of reliable integrating and non- integrating gene expression vectors, SBI delivers high-quality, cutting-edge products and services for mammalian gene delivery and expression. With products and services that have been used in thousands of peer-reviewed papers, SBI is your trusted partner for high-quality research. 04 06 12 14 PRODUCT INTEGRATING NON-INTEGRATING SERVICES SELECTOR VECTOR SYSTEMS VECTOR SYSTEMS Syn2Clone Lentiviral Enhanced Episomal Virus Packaging Vectors PiggyBac Cell Line Construction Transposon Minicircle Technology PhiC31 Integrase AAV PinPoint Targeted Integration Non-integrating Lentiviral Gene Knock-out Gene Knock-in Gene Edit AAVS1 Safe Harbor Site INDELS via NHEJ NN OR HR Donor HR Donor Vector Vector HR Donor Vector GENE INSERTION loxP loxP (with HR vector) Excision of GENE KNOCK-OUT selection cassette (with HR vector) CORRECTED GOI PRODUCT SELECTOR A WEALTH OF OPTIONS FOR GENE DELIVERY AND EXPRESSION Integrating Vectors With integrating vectors, the vector DNA becomes permanently incorporated into the genome, often at multiple copy numbers. Depending on the system, integration can be random or directed to a specific locus. -

World Resources Institute the Monsanto Company
World Resources Institute Sustainable Enterprise Program A program of the World Resources Institute The Monsanto Company: Quest for Sustainability (A) “Biotechnology represents a potentially sustainable For more than a decade, WRI's solution to the issue, not only of feeding people, but of providing Sustainable Enterprise Program (SEP) the economic growth that people are going to need to escape has harnessed the power of business to poverty…… [Biotechnology] poses the possibility of create profitable solutions to leapfrogging the industrial revolution and moving to a post- environment and development industrial society that is not only economically attractive, but challenges. BELL, a project of SEP, is also environmentally sustainable.i ” focused on working with managers and academics to make companies --Robert Shapiro, CEO, Monsanto Company more competitive by approaching social and environmental challenges as unmet market needs that provide Upon his promotion to CEO of chemical giant The business growth opportunities through Monsanto Company in 1995, Robert Shapiro became a vocal entrepreneurship, innovation, and champion of sustainable development and sought to redefine the organizational change. firm’s business strategy along principles of sustainability. Shapiro’s rhetoric was compelling. He captured analysts’ Permission to reprint this case is attention with the specter of mass hunger and environmental available at the BELL case store. degradation precipitated by rapid population growth and the Additional information on the Case -

Quick and Efficient Method for Genetic Transformation of Biopolymer
Technical Note Received: 29 July 2009 Revised: 14 September 2009 Accepted: 14 September 2009 Published online in Wiley Interscience: 29 October 2009 (www.interscience.wiley.com) DOI 10.1002/jctb.2284 Quick and efficient method for genetic transformation of biopolymer-producing bacteria Qin Wang,a Alexander P. Mueller,a Chean Ring Leong,b Ken’ichiro Matsumoto,b Seiichi Taguchib and Christopher T. Nomuraa∗ Abstract In order to genetically modify microorganisms capable of producing polyhydroxyalkanoate (PHA) biopolymers, a simple and rapid method to prepare freshly plated Pseudomonas cells for transformation via electroporation was developed. This method can be used to transfer both replicative plasmids and linear DNA to knock out genes into the cells. The transformation efficiencies were in the range of ≥107 transformants µg−1 DNA for replicative plasmids and ≥106 transformants µg−1 DNA for linear DNA, which are comparable with commercially available competent cells. Furthermore, this transformation procedure can be performed in less than 10 min, saving a great deal of time compared with traditional methods. Knockout mutants of several Pseudomonas species were generated by transformation of linear DNA and these mutations were verified by PCR and analysis of PHA content. c 2009 Society of Chemical Industry Keywords: transformation; electroporation; Pseudomonas putida; polyhydroxyalkanoates (PHAs) INTRODUCTION using various strains of P. putida.StrainsweregrowninLuria- Pseudomonas putida is a Gram-negative soil bacterium that plays Bertani (LB) medium (1% tryptone, 0.5% yeast extract, and 0.5% animportantroleinelementcycling innature,bioremediation,and NaCl) with the appropriate antibiotic when necessary. For selection production of polyhydroxyalkanoates (PHAs), which are environ- of transformants, kanamycin (Km) and gentamycin (Gm) were mentally friendly biodegradable plastics.1–3 Despite having a fully added to LB agar plates and liquid media at final concentrations sequenced genome,3 the functions of many ORFs in this organ- of 50 µgmL−1 and 20 µgmL−1, respectively. -

ES Cell Targeting Handbook
TABLE OF CONTENTS Overview of ES Core Facility Introduction Generation of Gene-Targeted ES Cells Karyotyping of Positive ES Clones ES Cell Request Form General Information for the Generation of Targeted Cells Principles of Gene Targeting Requirements for the Design of Targeting Constructs Screening Assay for the Identification of Targeted ES Clones Overview of ES Cell Culture ES Cell Factors Affecting Successful Chimera Production FAQ Overview of ES Core Facility Our Mission The ES Core Facility (ECF) was founded by the NINDS Core Center Grant and was established to benefit the contributors of this proposal. The mission of ECF is to effectively produce ES cell lines with a high probability of germline transmission. Core Service Services provided by the Core for a typical project include: • Provide guidance on the design of targeting construct • Generate targeted ES cell lines for the production of chimeric mice • Karyotyping ES cells to be micro-injected into blastocysts Consultation is available from ECF directors and staff members on the entire procedures of generating gene knock-out mice. Application for Service Prior to the initiation of a project, a brief meeting is generally required between the investigator and ECF facility staff resulting in a mutually acceptable research strategy. This strategy will outline specifics of the project including knockout strategy, KO construct design, screening assays, and other procedural issues relevant to the generation of targeted ES cells. In addition, a completed service application form, signed by the principal investigator and approved by the Core Director, will also be required. The Core Director will prioritize the service requests according to the difficulty of the project and work load. -

Intramuscular Electroporation Delivery of IFN- Gene Therapy for Inhibition of Tumor Growth Located at a Distant Site
Gene Therapy (2001) 8, 400–407 2001 Nature Publishing Group All rights reserved 0969-7128/01 $15.00 www.nature.com/gt RESEARCH ARTICLE Intramuscular electroporation delivery of IFN-␣ gene therapy for inhibition of tumor growth located at a distant site S Li, X Zhang, X Xia, L Zhou, R Breau, J Suen and E Hanna Department of Otolaryngology/Head and Neck Surgery, University of Arkansas School of Medicine, 4001 W Capital Avenue, Little Rock, AR 72205, USA Although electroporation has been shown in recent years to 2 or endostatin gene, also delivered by electro-injection. The be a powerful method for delivering genes to muscle, no increased therapeutic efficacy was associated with a high gene therapy via electro-injection has been studied for the level and extended duration of IFN-␣ expression in muscle treatment of tumors. In an immunocompetent tumor-bearing and serum. We also discovered that the high level of IFN-␣ murine model, we have found that delivery of a low dose of expression correlated with increased expression levels of reporter gene DNA (10 g) to muscle via electroporation the antiangiogenic genes IP-10 and Mig in local tumor under specific pulse conditions (two 25-ms pulses of 375 tissue, which may have led to the reduction of blood vessels V/cm) increased the level of gene expression by two logs of observed at the local tumor site. Delivery of increasing doses magnitude. Moreover, administration of 10 g of interferon (10–100 g) of IFN-␣ plasmid DNA by injection alone did (IFN)-␣ DNA plasmid using these parameters once a week not increase antitumor activity, whereas electroporation for 3 weeks increased the survival time and reduced squam- delivery of increasing doses (10–40 g) of IFN-␣ plasmid ous cell carcinoma (SCC) growth at a distant site in the DNA did increase the survival time. -

Delivery Strategies of the CRISPR-Cas9 Gene-Editing System for Therapeutic MARK Applications
Journal of Controlled Release 266 (2017) 17–26 Contents lists available at ScienceDirect Journal of Controlled Release journal homepage: www.elsevier.com/locate/jconrel Review article Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic MARK applications ⁎ Chang Liu, Li Zhang, Hao Liu, Kun Cheng Division of Pharmaceutical Sciences, School of Pharmacy, University of Missouri-Kansas City, Kansas City, MO 64108, United States ARTICLE INFO ABSTRACT Keywords: The CRISPR-Cas9 genome-editing system is a part of the adaptive immune system in archaea and bacteria to CRISPR-Cas9 defend against invasive nucleic acids from phages and plasmids. The single guide RNA (sgRNA) of the system Delivery recognizes its target sequence in the genome, and the Cas9 nuclease of the system acts as a pair of scissors to Gene-editing cleave the double strands of DNA. Since its discovery, CRISPR-Cas9 has become the most robust platform for Gene therapy genome engineering in eukaryotic cells. Recently, the CRISPR-Cas9 system has triggered enormous interest in Non-viral delivery therapeutic applications. CRISPR-Cas9 can be applied to correct disease-causing gene mutations or engineer T Nanoparticle cells for cancer immunotherapy. The first clinical trial using the CRISPR-Cas9 technology was conducted in 2016. Despite the great promise of the CRISPR-Cas9 technology, several challenges remain to be tackled before its successful applications for human patients. The greatest challenge is the safe and efficient delivery of the CRISPR-Cas9 genome-editing system to target cells in human body. In this review, we will introduce the mo- lecular mechanism and different strategies to edit genes using the CRISPR-Cas9 system. -

Human Inheritable Genetic Modifications
Human Inheritable Genetic Modifications Assessing Scientific, Ethical, Religious, and Policy Issues Prepared by the American Association for the Advancement of Science Mark S. Frankel Audrey R. Chapman September 2000 http://www.aaas.org/spp/dspp/sfrl/germline/main.htm i This report is the product of a collaboration between the authors and a working group convened to advise the authors, and does not necessarily represent the views of American Association for the Advancement of Science or The Greenwall Foundation, which funded this study. Copyright © 2000 American Association for the Advancement of Science Cover: Designed and created by the Office of Publication Services at the American Association for the Advancement of Science. ii Table of Contents Acknowledgements…………………………………………………v Introduction………………………………………………………….1 Major Findings, Concerns, and Recommendations…………………7 Defining Inheritable Genetic Modific ation……………….………..11 Therapeutic Need…………………………………………………..13 Efficacy of Different Approaches to IGM…………………………15 Safety Issues……………………………………………………….23 Inadvertent Germ Line Modific ation………………………………26 Religious Perspectives……………………………………………..27 Ethical Analysis and Considerations……………………………….32 Ethically Appropriate Applications of IGM: Therapy versus Enhancement.………………………………………………………40 Reproductive Rights………………………………………………..44 Balancing Scientific Freedom and Responsibility…………………45 Oversight…………………………………………………………...46 Conclusion.…………………………………………………………56 Glossary…………………………………………………………….59 Appendix A: AAAS Working Group Members……………………65 -
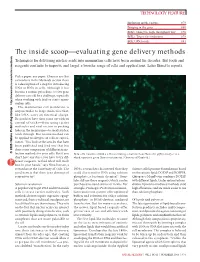
The Inside Scoop—Evaluating Gene Delivery Methods
TECHNOLOGY FEATURE Exploiting mother nature 878 Bringing in the guns 880 BOX 1: Going the high-throughput way 876 BOX 2: Target: the endosome 878 BOX 3: RNAi craze 881 The inside scoop⎯evaluating gene delivery methods Techniques for delivering nucleic acids into mammalian cells have been around for decades. But tools and methods reagents continue to improve and target a broader range of cells and applications. Laura Bonetta reports. Pick a paper, any paper. Chances are that somewhere in the Methods section there .com/nature e is a description of a step for introducing DNA or RNA in cells. Although it has .natur w become a routine procedure, in vitro gene delivery can still be a challenge, especially when working with frail or scarce mam- http://ww malian cells. The mammalian cell membrane is oup impenetrable to large molecules that, r G like DNA, carry an electrical charge. Researchers have thus come up with an arsenal of tricks⎯from using carrier lishing molecules and viral vectors to pocking b holes in the membrane⎯to sneak nucleic Pu acids through. But no one method can be applied to all types of cells or experi- Nature ments. “You look at the articles that have 5 been published and find one that has 200 done some comparison of different trans- © fection methods for your cells. But if you HeLa cells transduced with a self-inactivating retrovirus from Clontech’s pQX (retroQ) series, don’t have any clues, you have to try dif- which expresses green fluorescent protein. (Courtesy of Clontech.) ferent reagents, to find what will work best in your hands,” says Nina Iversen, a researcher at the University of Oslo. -

Engineering of Primary Human B Cells with CRISPR/Cas9 Targeted Nuclease Received: 26 January 2018 Matthew J
www.nature.com/scientificreports OPEN Engineering of Primary Human B cells with CRISPR/Cas9 Targeted Nuclease Received: 26 January 2018 Matthew J. Johnson1,2,3, Kanut Laoharawee1,2,3, Walker S. Lahr1,2,3, Beau R. Webber1,2,3 & Accepted: 23 July 2018 Branden S. Moriarity1,2,3 Published: xx xx xxxx B cells ofer unique opportunities for gene therapy because of their ability to secrete large amounts of protein in the form of antibody and persist for the life of the organism as plasma cells. Here, we report optimized CRISPR/Cas9 based genome engineering of primary human B cells. Our procedure involves enrichment of CD19+ B cells from PBMCs followed by activation, expansion, and electroporation of CRISPR/Cas9 reagents. We are able expand total B cells in culture 10-fold and outgrow the IgD+ IgM+ CD27− naïve subset from 35% to over 80% of the culture. B cells are receptive to nucleic acid delivery via electroporation 3 days after stimulation, peaking at Day 7 post stimulation. We tested chemically modifed sgRNAs and Alt-R gRNAs targeting CD19 with Cas9 mRNA or Cas9 protein. Using this system, we achieved genetic and protein knockout of CD19 at rates over 70%. Finally, we tested sgRNAs targeting the AAVS1 safe harbor site using Cas9 protein in combination with AAV6 to deliver donor template encoding a splice acceptor-EGFP cassette, which yielded site-specifc integration frequencies up to 25%. The development of methods for genetically engineered B cells opens the door to a myriad of applications in basic research, antibody production, and cellular therapeutics. -

Cutting Eugenics out of CRISPR-Cas9
Ethics in Biology, Engineering & Medicine - An International Journal, 6(3–4): 263–279 (2015) Cutting Eugenics Out of CRISPR-Cas9 Carolyn Brokowski,a,* Marya Pollack,b & Robert Pollackc aBioethics (Medical Ethics) Department, Columbia University School of Professional Studies, New York, New York; bDepartment of Psychiatry, Columbia University College of Physicians and Surgeons, Inwood Clinic, New York, New York; cBiological Sciences Department, Columbia University School of the Arts, New York, New York *Address all correspondence to: Carolyn Brokowski, M.S. Candidate; Bioethics (Medical Ethics) Department, Columbia University School of Professional Studies, 203 Lewisohn Hall, 2970 Broadway, MC 4119, New York, NY 10027; E-mail: [email protected] ABSTRACT: The use of clustered regularly interspaced short palindromic repeats (CRISPR) and their associated (Cas) proteins (the CRISPR-Cas system) in genomic engineering is among the most promising biomedical innovations to occur in the last few decades. One of this system’s most profound features is its ability to edit genomes with impressive specificity, which may cause significant alterations of cellular, tissue, and organismal phenotypes at the near instance of the editing, over the lifespan of the organism and potentially into any number of future genera- tions. We argue that the use of the CRISPR-Cas9 system to edit the human germline should be legally prohibited on account of the system’s potential for generating an unjust eugenic future. Its use in nongermline experimentation and applications, however, should not be constrained on eugenic grounds. Such a blanket legal prohibition might limit the progress gleaned from this technology. Allowing experimentation in human subjects more broadly might expose par- ticipants to considerable risk and potentially harmful outcomes, and the system might prove unable to realize tangible therapeutic outcomes that seem likely ex ante. -

An Update on the Tools for Creating Transgenic Animal Models of Human Diseases – Focus on Atherosclerosis
Brazilian Journal of Medical and Biological Research (2019) 52(5): e8108, http://dx.doi.org/10.1590/1414-431X20198108 ISSN 1414-431X Review 1/7 An update on the tools for creating transgenic animal models of human diseases – focus on atherosclerosis A.S. Volobueva2, A.N. Orekhov 1,3,4, and A.V. Deykin 1 1Institute of Gene Biology, Russian Academy of Sciences, Moscow, Russia 2Laboratory of Gene Therapy, Biocad Biotechnology Company, Strelnya, Russia 3Laboratory of Angiopathology, Institute of General Pathology and Pathophysiology, Moscow, Russia 4Institute for Atherosclerosis Research, Skolkovo Innovative Center, Moscow, Russia Abstract Animal models of diseases are invaluable tools of modern medicine. More than forty years have passed since the first successful experiments and the spectrum of available models, as well as the list of methods for creating them, have expanded dramatically. The major step forward in creating specific disease models was the development of gene editing techniques, which allowed for targeted modification of the animal’s genome. In this review, we discuss the available tools for creating transgenic animal models, such as transgenesis methods, recombinases, and nucleases, including zinc finger nuclease (ZFN), transcription activator-like effector nuclease (TALEN), and CRISPR/Cas9 systems. We then focus specifically on the models of atherosclerosis, especially mouse models that greatly contributed to improving our understanding of the disease pathogenesis and we outline their characteristics and limitations. Key words: Animal models; Gene editing; Atherosclerosis Introduction Model organisms are widely used in biomedicine reliability of resulting models. Moreover, uncontrolled gene for studying the pathophysiology of human diseases and expression could affect multiple pathways in the animal developing novel therapeutic and diagnostic methods.