An Update on the Tools for Creating Transgenic Animal Models of Human Diseases – Focus on Atherosclerosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

REVIEW Gene Therapy
Leukemia (2001) 15, 523–544 2001 Nature Publishing Group All rights reserved 0887-6924/01 $15.00 www.nature.com/leu REVIEW Gene therapy: principles and applications to hematopoietic cells VFI Van Tendeloo1,2, C Van Broeckhoven2 and ZN Berneman1 1Laboratory of Experimental Hematology, University of Antwerp (UIA), Antwerp University Hospital (UZA), Antwerp; and 2Laboratory of Molecular Genetics, University of Antwerp (UIA), Department of Molecular Genetics, Flanders Interuniversity Institute for Biotechnology (VIB), Antwerp, Belgium Ever since the development of technology allowing the transfer Recombinant viral vectors of new genes into eukaryotic cells, the hematopoietic system has been an obvious and desirable target for gene therapy. The last 10 years have witnessed an explosion of interest in this Biological gene transfer methods make use of modified DNA approach to treat human disease, both inherited and acquired, or RNA viruses to infect the cell, thereby introducing and with the initiation of multiple clinical protocols. All gene ther- expressing its genome which contains the gene of interest (= apy strategies have two essential technical requirements. ‘transduction’).1 The most commonly used viral vectors are These are: (1) the efficient introduction of the relevant genetic discussed below. In each case, recombinant viruses have had material into the target cell and (2) the expression of the trans- gene at therapeutic levels. Conceptual and technical hurdles the genes encoding essential replicative and/or packaging pro- involved with these requirements are still the objects of active teins replaced by the gene of interest. Advantages and disad- research. To date, the most widely used and best understood vantages of each recombinant viral vector are summarized in vectors for gene transfer in hematopoietic cells are derived Table 1. -

Improving Treatment of Genetic Diseases with Crispr-Cas9 Rna-Guided Genome Editing
Sanchez 3:00 Team R06 Disclaimer: This paper partially fulfills a writing requirement for first-year (freshmen) engineering students at the University of Pittsburgh Swanson School of Engineering. This paper is a student paper, not a professional paper. This paper is not intended for publication or public circulation. This paper is based on publicly available information, and while this paper might contain the names of actual companies, products, and people, it cannot and does not contain all relevant information/data or analyses related to companies, products, and people named. All conclusions drawn by the authors are the opinions of the authors, first- year (freshmen) students completing this paper to fulfill a university writing requirement. If this paper or the information therein is used for any purpose other than the authors' partial fulfillment of a writing requirement for first-year (freshmen) engineering students at the University of Pittsburgh Swanson School of Engineering, the users are doing so at their own--not at the students', at the Swanson School's, or at the University of Pittsburgh's--risk. IMPROVING TREATMENT OF GENETIC DISEASES WITH CRISPR-CAS9 RNA-GUIDED GENOME EDITING Arijit Dutta [email protected] , Benjamin Ahlmark [email protected], Nate Majer [email protected] Abstract—Genetic illnesses are among the most difficult to treat as it is challenging to safely and effectively alter DNA. INTRODUCTION: THE WHAT, WHY, AND DNA is the basic code for all hereditary traits, so any HOW OF CRISPR-CAS9 alteration to DNA risks fundamentally altering the way someone’s genes are expressed. This change could lead to What Is CRISPR-Cas9? unintended consequences for both the individual whose DNA was altered and any offspring they may have in the future, CRISPR-Cas9 is an acronym that stands for “Clustered compounding the risk. -

Gene Delivery & Expression
GENE DELIVERY & EXPRESSION GENE DELIVERY & EXPRESSION LENTIVIRUS, PIGGYBAC, MINICIRCLES, AND MORE SYSTEMBIO.COM SYSTEMS FOR ANY APPLICATION: LENTIVIRAL TOOLS AND MORE In today’s busy labs, getting experiments to work right the first time is critical—not only do you want results you can trust, you want them fast. Which is why SBI has spent over a decade developing a range of exceptionally high-performing products for gene delivery and expression. From our popular high-titer lentivirus production tools to the latest in AAV-based gene delivery systems and a range of reliable integrating and non- integrating gene expression vectors, SBI delivers high-quality, cutting-edge products and services for mammalian gene delivery and expression. With products and services that have been used in thousands of peer-reviewed papers, SBI is your trusted partner for high-quality research. 04 06 12 14 PRODUCT INTEGRATING NON-INTEGRATING SERVICES SELECTOR VECTOR SYSTEMS VECTOR SYSTEMS Syn2Clone Lentiviral Enhanced Episomal Virus Packaging Vectors PiggyBac Cell Line Construction Transposon Minicircle Technology PhiC31 Integrase AAV PinPoint Targeted Integration Non-integrating Lentiviral Gene Knock-out Gene Knock-in Gene Edit AAVS1 Safe Harbor Site INDELS via NHEJ NN OR HR Donor HR Donor Vector Vector HR Donor Vector GENE INSERTION loxP loxP (with HR vector) Excision of GENE KNOCK-OUT selection cassette (with HR vector) CORRECTED GOI PRODUCT SELECTOR A WEALTH OF OPTIONS FOR GENE DELIVERY AND EXPRESSION Integrating Vectors With integrating vectors, the vector DNA becomes permanently incorporated into the genome, often at multiple copy numbers. Depending on the system, integration can be random or directed to a specific locus. -

Strategies for Precision Modulation of Gene Expression by Epigenome Editing: an Overview Benjamin I
Laufer and Singh Epigenetics & Chromatin (2015) 8:34 DOI 10.1186/s13072-015-0023-7 REVIEW Open Access Strategies for precision modulation of gene expression by epigenome editing: an overview Benjamin I. Laufer* and Shiva M. Singh Abstract Genome editing technology has evolved rather quickly and become accessible to most researchers. It has resulted in far reaching implications and a number of novel designer systems including epigenome editing. Epigenome editing utilizes a combination of nuclease-null genome editing systems and effector domains to modulate gene expression. In particular, Zinc Finger, Transcription-Activator-Like Effector, and CRISPR/Cas9 have emerged as modular systems that can be modified to allow for precision manipulation of epigenetic marks without altering underlying DNA sequence. This review contains a comprehensive catalog of effector domains that can be used with components of genome editing systems to achieve epigenome editing. Ultimately, the evidence-based design of epigenome editing offers a novel improvement to the limited attenuation strategies. There is much potential for editing and/or correcting gene expression in somatic cells toward a new era of functional genomics and personalized medicine. Keywords: Regulation of gene expression, Functional genomics, Stem cells, dCas9, CRISPR/Cas9, Zinc Finger, Transcription-Activator-Like Effector (TALE), Synthetic biology Background editing systems rely on two components, a DNA-bind- The modulation of gene expression can be achieved by ing element, and nuclease, to modify the targeted DNA a variety of biotechnologies such as RNA interference, sequence. Genome editing can be used to study protein non-precision drugs, and artificial transcription factors function by altering coding sequence or achieve tran- (ATFs). -

CRISPR Foki Dead Cas9 System: Principles and Applications in Genome Engineering
cells Review CRISPR FokI Dead Cas9 System: Principles and Applications in Genome Engineering Maryam Saifaldeen y, Dana E. Al-Ansari y , Dindial Ramotar * and Mustapha Aouida * College of Health and Life Sciences, Division of Biological and Biomedical Sciences, Hamad Bin Khalifa University, Education City, Qatar Foundation, Doha P.O.Box 34110, Qatar; [email protected] (M.S.); [email protected] (D.E.A.-A.) * Correspondence: [email protected] (D.R.); [email protected] (M.A.) These authors contributed equally to this work. y Received: 27 September 2020; Accepted: 19 November 2020; Published: 21 November 2020 Abstract: The identification of the robust clustered regularly interspersed short palindromic repeats (CRISPR) associated endonuclease (Cas9) system gene-editing tool has opened up a wide range of potential therapeutic applications that were restricted by more complex tools, including zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs). Nevertheless, the high frequency of CRISPR system off-target activity still limits its applications, and, thus, advanced strategies for highly specific CRISPR/Cas9-mediated genome editing are continuously under development including CRISPR–FokI dead Cas9 (fdCas9). fdCas9 system is derived from linking a FokI endonuclease catalytic domain to an inactive Cas9 protein and requires a pair of guide sgRNAs that bind to the sense and antisense strands of the DNA in a protospacer adjacent motif (PAM)-out orientation, with a defined spacer sequence range around the target site. The dimerization of FokI domains generates DNA double-strand breaks, which activates the DNA repair machinery and results in genomic edit. So far, all the engineered fdCas9 variants have shown promising gene-editing activities in human cells when compared to other platforms. -

Delivery Strategies of the CRISPR-Cas9 Gene-Editing System for Therapeutic MARK Applications
Journal of Controlled Release 266 (2017) 17–26 Contents lists available at ScienceDirect Journal of Controlled Release journal homepage: www.elsevier.com/locate/jconrel Review article Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic MARK applications ⁎ Chang Liu, Li Zhang, Hao Liu, Kun Cheng Division of Pharmaceutical Sciences, School of Pharmacy, University of Missouri-Kansas City, Kansas City, MO 64108, United States ARTICLE INFO ABSTRACT Keywords: The CRISPR-Cas9 genome-editing system is a part of the adaptive immune system in archaea and bacteria to CRISPR-Cas9 defend against invasive nucleic acids from phages and plasmids. The single guide RNA (sgRNA) of the system Delivery recognizes its target sequence in the genome, and the Cas9 nuclease of the system acts as a pair of scissors to Gene-editing cleave the double strands of DNA. Since its discovery, CRISPR-Cas9 has become the most robust platform for Gene therapy genome engineering in eukaryotic cells. Recently, the CRISPR-Cas9 system has triggered enormous interest in Non-viral delivery therapeutic applications. CRISPR-Cas9 can be applied to correct disease-causing gene mutations or engineer T Nanoparticle cells for cancer immunotherapy. The first clinical trial using the CRISPR-Cas9 technology was conducted in 2016. Despite the great promise of the CRISPR-Cas9 technology, several challenges remain to be tackled before its successful applications for human patients. The greatest challenge is the safe and efficient delivery of the CRISPR-Cas9 genome-editing system to target cells in human body. In this review, we will introduce the mo- lecular mechanism and different strategies to edit genes using the CRISPR-Cas9 system. -

Human Inheritable Genetic Modifications
Human Inheritable Genetic Modifications Assessing Scientific, Ethical, Religious, and Policy Issues Prepared by the American Association for the Advancement of Science Mark S. Frankel Audrey R. Chapman September 2000 http://www.aaas.org/spp/dspp/sfrl/germline/main.htm i This report is the product of a collaboration between the authors and a working group convened to advise the authors, and does not necessarily represent the views of American Association for the Advancement of Science or The Greenwall Foundation, which funded this study. Copyright © 2000 American Association for the Advancement of Science Cover: Designed and created by the Office of Publication Services at the American Association for the Advancement of Science. ii Table of Contents Acknowledgements…………………………………………………v Introduction………………………………………………………….1 Major Findings, Concerns, and Recommendations…………………7 Defining Inheritable Genetic Modific ation……………….………..11 Therapeutic Need…………………………………………………..13 Efficacy of Different Approaches to IGM…………………………15 Safety Issues……………………………………………………….23 Inadvertent Germ Line Modific ation………………………………26 Religious Perspectives……………………………………………..27 Ethical Analysis and Considerations……………………………….32 Ethically Appropriate Applications of IGM: Therapy versus Enhancement.………………………………………………………40 Reproductive Rights………………………………………………..44 Balancing Scientific Freedom and Responsibility…………………45 Oversight…………………………………………………………...46 Conclusion.…………………………………………………………56 Glossary…………………………………………………………….59 Appendix A: AAAS Working Group Members……………………65 -
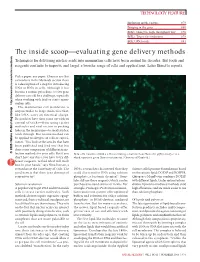
The Inside Scoop—Evaluating Gene Delivery Methods
TECHNOLOGY FEATURE Exploiting mother nature 878 Bringing in the guns 880 BOX 1: Going the high-throughput way 876 BOX 2: Target: the endosome 878 BOX 3: RNAi craze 881 The inside scoop⎯evaluating gene delivery methods Techniques for delivering nucleic acids into mammalian cells have been around for decades. But tools and methods reagents continue to improve and target a broader range of cells and applications. Laura Bonetta reports. Pick a paper, any paper. Chances are that somewhere in the Methods section there .com/nature e is a description of a step for introducing DNA or RNA in cells. Although it has .natur w become a routine procedure, in vitro gene delivery can still be a challenge, especially when working with frail or scarce mam- http://ww malian cells. The mammalian cell membrane is oup impenetrable to large molecules that, r G like DNA, carry an electrical charge. Researchers have thus come up with an arsenal of tricks⎯from using carrier lishing molecules and viral vectors to pocking b holes in the membrane⎯to sneak nucleic Pu acids through. But no one method can be applied to all types of cells or experi- Nature ments. “You look at the articles that have 5 been published and find one that has 200 done some comparison of different trans- © fection methods for your cells. But if you HeLa cells transduced with a self-inactivating retrovirus from Clontech’s pQX (retroQ) series, don’t have any clues, you have to try dif- which expresses green fluorescent protein. (Courtesy of Clontech.) ferent reagents, to find what will work best in your hands,” says Nina Iversen, a researcher at the University of Oslo. -

Zinc Finger Nucleases (Zfns) and Transcription Activator-Like Effector Nucleases (Talens) Deepak Reyon Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2011 Genome engineering using DNA-binding proteins: zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs) Deepak Reyon Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Bioinformatics Commons, Computational Biology Commons, and the Genomics Commons Recommended Citation Reyon, Deepak, "Genome engineering using DNA-binding proteins: zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs)" (2011). Graduate Theses and Dissertations. 14745. https://lib.dr.iastate.edu/etd/14745 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Genome engineering using DNA-binding proteins: zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs) by Deepak Reyon A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Bioinformatics and Computational Biology Program of Study Committee: Drena Dobbs, Co-Major Professor Edward Yu, Co-Major Professor Vasant Honavar Amy Andreotti Gaya Amarasinghe Iowa State University Ames, -

A Revolution Toward Gene-Editing Technology and Its Application to Crop Improvement
International Journal of Molecular Sciences Review A Revolution toward Gene-Editing Technology and Its Application to Crop Improvement Sunny Ahmar 1, Sumbul Saeed 1, Muhammad Hafeez Ullah Khan 1 , Shahid Ullah Khan 1 , Freddy Mora-Poblete 2 , Muhammad Kamran 3 , Aroosha Faheem 4, Ambreen Maqsood 5 , Muhammad Rauf 6 , Saba Saleem 7, Woo-Jong Hong 8 and Ki-Hong Jung 8,* 1 College of Plant Sciences and Technology Huazhong Agricultural University, Wuhan 430070, China; [email protected] (S.A.); [email protected] (S.S.); [email protected] (M.H.U.K.); [email protected] (S.U.K.) 2 Institute of Biological Sciences, University of Talca, 2 Norte 685, Talca 3460000, Chile; [email protected] 3 Key Laboratory of Arable Land Conservation (Middle and Lower Reaches of Yangtze River), Ministry of Agriculture, College of Resources and Environment, Huazhong Agricultural University, Wuhan 430070, China; [email protected] 4 Sate Key Laboratory of Agricultural Microbiology and State Key Laboratory of Microbial Biosensor, College of Life Sciences Huazhong Agriculture University Wuhan, Wuhan 430070, China; [email protected] 5 Department of Plant Pathology, University College of Agriculture and Environmental Sciences, The Islamia University of Bahawalpur, Bahawalpur 63100, Pakistan; [email protected] 6 National Institute for Biotechnology and Genetic Engineering (NIBGE), Faisalabad 38000, Pakistan; [email protected] 7 Department of Bioscience, COMSATS Institute of Information Technology, Islamabad 45550, Pakistan; [email protected] 8 Graduate School of Biotechnology & Crop Biotech Institute, Kyung Hee University, Yongin 17104, Korea; [email protected] * Correspondence: [email protected] Received: 14 July 2020; Accepted: 5 August 2020; Published: 7 August 2020 Abstract: Genome editing is a relevant, versatile, and preferred tool for crop improvement, as well as for functional genomics. -

Gene Delivery in Mouse Auditory Brainstem and Hindbrain Using in Utero Electroporation Laurence S David, Jamila Aitoubah, Lee Stephen Lesperance and Lu-Yang Wang*
David et al. Molecular Brain 2014, 7:51 http://www.molecularbrain.com/content/7/1/51 METHODOLOGY Open Access Gene delivery in mouse auditory brainstem and hindbrain using in utero electroporation Laurence S David, Jamila Aitoubah, Lee Stephen Lesperance and Lu-Yang Wang* Abstract Background: Manipulation of gene expression via recombinant viral vectors and creation of transgenic knock-out/ in animals has revolutionized our understanding of genes that play critical roles during neuronal development and pathophysiology of neurological disorders. Recently, target-specific genetic manipulations are made possible to perform in combination with specific Cre-lines, albeit costly, labor-intensive and time consuming. Thus, alternative methods of gene manipulations to address important biological questions are highly desirable. In this study, we utilized in utero electroporation technique which involves efficient delivery of hindbrain-specific enhancer/promoter construct, Krox20 into the third ventricle of live mouse embryo to investigate green fluorescent protein (GFP) expression pattern in mouse auditory brainstem and other hindbrain neurons. Results: We created a GFP/DNA construct containing a Krox20 B enhancer and β-globin promoter to drive GFP expression in the hindbrain via injection into the third ventricle of E12 to E13.5 mice. Electrical currents were applied directly to the embryonic hindbrain to allow DNA uptake into the cell. Confocal images were then acquired from fixed brain slices to analyze GFP expression in mouse whole brain at different postnatal stages (P6-P21). By using a cell-type specific enhancer as well as region specific injection and electroporation, robust GFP expression in the cerebellum and auditory brainstem but not in the forebrain was observed. -

Designer Nucleases to Treat Malignant Cancers Driven by Viral Oncogenes Tristan A
Scott and Morris Virol J (2021) 18:18 https://doi.org/10.1186/s12985-021-01488-1 REVIEW Open Access Designer nucleases to treat malignant cancers driven by viral oncogenes Tristan A. Scott* and Kevin V. Morris Abstract Viral oncogenic transformation of healthy cells into a malignant state is a well-established phenomenon but took decades from the discovery of tumor-associated viruses to their accepted and established roles in oncogenesis. Viruses cause ~ 15% of know cancers and represents a signifcant global health burden. Beyond simply causing cel- lular transformation into a malignant form, a number of these cancers are augmented by a subset of viral factors that signifcantly enhance the tumor phenotype and, in some cases, are locked in a state of oncogenic addiction, and sub- stantial research has elucidated the mechanisms in these cancers providing a rationale for targeted inactivation of the viral components as a treatment strategy. In many of these virus-associated cancers, the prognosis remains extremely poor, and novel drug approaches are urgently needed. Unlike non-specifc small-molecule drug screens or the broad- acting toxic efects of chemo- and radiation therapy, the age of designer nucleases permits a rational approach to inactivating disease-causing targets, allowing for permanent inactivation of viral elements to inhibit tumorigenesis with growing evidence to support their efcacy in this role. Although many challenges remain for the clinical applica- tion of designer nucleases towards viral oncogenes; the uniqueness and clear molecular mechanism of these targets, combined with the distinct advantages of specifc and permanent inactivation by nucleases, argues for their develop- ment as next-generation treatments for this aggressive group of cancers.