Gene Delivery & Expression
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

REVIEW Gene Therapy
Leukemia (2001) 15, 523–544 2001 Nature Publishing Group All rights reserved 0887-6924/01 $15.00 www.nature.com/leu REVIEW Gene therapy: principles and applications to hematopoietic cells VFI Van Tendeloo1,2, C Van Broeckhoven2 and ZN Berneman1 1Laboratory of Experimental Hematology, University of Antwerp (UIA), Antwerp University Hospital (UZA), Antwerp; and 2Laboratory of Molecular Genetics, University of Antwerp (UIA), Department of Molecular Genetics, Flanders Interuniversity Institute for Biotechnology (VIB), Antwerp, Belgium Ever since the development of technology allowing the transfer Recombinant viral vectors of new genes into eukaryotic cells, the hematopoietic system has been an obvious and desirable target for gene therapy. The last 10 years have witnessed an explosion of interest in this Biological gene transfer methods make use of modified DNA approach to treat human disease, both inherited and acquired, or RNA viruses to infect the cell, thereby introducing and with the initiation of multiple clinical protocols. All gene ther- expressing its genome which contains the gene of interest (= apy strategies have two essential technical requirements. ‘transduction’).1 The most commonly used viral vectors are These are: (1) the efficient introduction of the relevant genetic discussed below. In each case, recombinant viruses have had material into the target cell and (2) the expression of the trans- gene at therapeutic levels. Conceptual and technical hurdles the genes encoding essential replicative and/or packaging pro- involved with these requirements are still the objects of active teins replaced by the gene of interest. Advantages and disad- research. To date, the most widely used and best understood vantages of each recombinant viral vector are summarized in vectors for gene transfer in hematopoietic cells are derived Table 1. -

Delivery Strategies of the CRISPR-Cas9 Gene-Editing System for Therapeutic MARK Applications
Journal of Controlled Release 266 (2017) 17–26 Contents lists available at ScienceDirect Journal of Controlled Release journal homepage: www.elsevier.com/locate/jconrel Review article Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic MARK applications ⁎ Chang Liu, Li Zhang, Hao Liu, Kun Cheng Division of Pharmaceutical Sciences, School of Pharmacy, University of Missouri-Kansas City, Kansas City, MO 64108, United States ARTICLE INFO ABSTRACT Keywords: The CRISPR-Cas9 genome-editing system is a part of the adaptive immune system in archaea and bacteria to CRISPR-Cas9 defend against invasive nucleic acids from phages and plasmids. The single guide RNA (sgRNA) of the system Delivery recognizes its target sequence in the genome, and the Cas9 nuclease of the system acts as a pair of scissors to Gene-editing cleave the double strands of DNA. Since its discovery, CRISPR-Cas9 has become the most robust platform for Gene therapy genome engineering in eukaryotic cells. Recently, the CRISPR-Cas9 system has triggered enormous interest in Non-viral delivery therapeutic applications. CRISPR-Cas9 can be applied to correct disease-causing gene mutations or engineer T Nanoparticle cells for cancer immunotherapy. The first clinical trial using the CRISPR-Cas9 technology was conducted in 2016. Despite the great promise of the CRISPR-Cas9 technology, several challenges remain to be tackled before its successful applications for human patients. The greatest challenge is the safe and efficient delivery of the CRISPR-Cas9 genome-editing system to target cells in human body. In this review, we will introduce the mo- lecular mechanism and different strategies to edit genes using the CRISPR-Cas9 system. -

Human Inheritable Genetic Modifications
Human Inheritable Genetic Modifications Assessing Scientific, Ethical, Religious, and Policy Issues Prepared by the American Association for the Advancement of Science Mark S. Frankel Audrey R. Chapman September 2000 http://www.aaas.org/spp/dspp/sfrl/germline/main.htm i This report is the product of a collaboration between the authors and a working group convened to advise the authors, and does not necessarily represent the views of American Association for the Advancement of Science or The Greenwall Foundation, which funded this study. Copyright © 2000 American Association for the Advancement of Science Cover: Designed and created by the Office of Publication Services at the American Association for the Advancement of Science. ii Table of Contents Acknowledgements…………………………………………………v Introduction………………………………………………………….1 Major Findings, Concerns, and Recommendations…………………7 Defining Inheritable Genetic Modific ation……………….………..11 Therapeutic Need…………………………………………………..13 Efficacy of Different Approaches to IGM…………………………15 Safety Issues……………………………………………………….23 Inadvertent Germ Line Modific ation………………………………26 Religious Perspectives……………………………………………..27 Ethical Analysis and Considerations……………………………….32 Ethically Appropriate Applications of IGM: Therapy versus Enhancement.………………………………………………………40 Reproductive Rights………………………………………………..44 Balancing Scientific Freedom and Responsibility…………………45 Oversight…………………………………………………………...46 Conclusion.…………………………………………………………56 Glossary…………………………………………………………….59 Appendix A: AAAS Working Group Members……………………65 -
The Inside Scoop—Evaluating Gene Delivery Methods
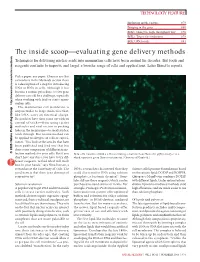
TECHNOLOGY FEATURE Exploiting mother nature 878 Bringing in the guns 880 BOX 1: Going the high-throughput way 876 BOX 2: Target: the endosome 878 BOX 3: RNAi craze 881 The inside scoop⎯evaluating gene delivery methods Techniques for delivering nucleic acids into mammalian cells have been around for decades. But tools and methods reagents continue to improve and target a broader range of cells and applications. Laura Bonetta reports. Pick a paper, any paper. Chances are that somewhere in the Methods section there .com/nature e is a description of a step for introducing DNA or RNA in cells. Although it has .natur w become a routine procedure, in vitro gene delivery can still be a challenge, especially when working with frail or scarce mam- http://ww malian cells. The mammalian cell membrane is oup impenetrable to large molecules that, r G like DNA, carry an electrical charge. Researchers have thus come up with an arsenal of tricks⎯from using carrier lishing molecules and viral vectors to pocking b holes in the membrane⎯to sneak nucleic Pu acids through. But no one method can be applied to all types of cells or experi- Nature ments. “You look at the articles that have 5 been published and find one that has 200 done some comparison of different trans- © fection methods for your cells. But if you HeLa cells transduced with a self-inactivating retrovirus from Clontech’s pQX (retroQ) series, don’t have any clues, you have to try dif- which expresses green fluorescent protein. (Courtesy of Clontech.) ferent reagents, to find what will work best in your hands,” says Nina Iversen, a researcher at the University of Oslo. -

An Update on the Tools for Creating Transgenic Animal Models of Human Diseases – Focus on Atherosclerosis
Brazilian Journal of Medical and Biological Research (2019) 52(5): e8108, http://dx.doi.org/10.1590/1414-431X20198108 ISSN 1414-431X Review 1/7 An update on the tools for creating transgenic animal models of human diseases – focus on atherosclerosis A.S. Volobueva2, A.N. Orekhov 1,3,4, and A.V. Deykin 1 1Institute of Gene Biology, Russian Academy of Sciences, Moscow, Russia 2Laboratory of Gene Therapy, Biocad Biotechnology Company, Strelnya, Russia 3Laboratory of Angiopathology, Institute of General Pathology and Pathophysiology, Moscow, Russia 4Institute for Atherosclerosis Research, Skolkovo Innovative Center, Moscow, Russia Abstract Animal models of diseases are invaluable tools of modern medicine. More than forty years have passed since the first successful experiments and the spectrum of available models, as well as the list of methods for creating them, have expanded dramatically. The major step forward in creating specific disease models was the development of gene editing techniques, which allowed for targeted modification of the animal’s genome. In this review, we discuss the available tools for creating transgenic animal models, such as transgenesis methods, recombinases, and nucleases, including zinc finger nuclease (ZFN), transcription activator-like effector nuclease (TALEN), and CRISPR/Cas9 systems. We then focus specifically on the models of atherosclerosis, especially mouse models that greatly contributed to improving our understanding of the disease pathogenesis and we outline their characteristics and limitations. Key words: Animal models; Gene editing; Atherosclerosis Introduction Model organisms are widely used in biomedicine reliability of resulting models. Moreover, uncontrolled gene for studying the pathophysiology of human diseases and expression could affect multiple pathways in the animal developing novel therapeutic and diagnostic methods. -

Gene Delivery in Mouse Auditory Brainstem and Hindbrain Using in Utero Electroporation Laurence S David, Jamila Aitoubah, Lee Stephen Lesperance and Lu-Yang Wang*
David et al. Molecular Brain 2014, 7:51 http://www.molecularbrain.com/content/7/1/51 METHODOLOGY Open Access Gene delivery in mouse auditory brainstem and hindbrain using in utero electroporation Laurence S David, Jamila Aitoubah, Lee Stephen Lesperance and Lu-Yang Wang* Abstract Background: Manipulation of gene expression via recombinant viral vectors and creation of transgenic knock-out/ in animals has revolutionized our understanding of genes that play critical roles during neuronal development and pathophysiology of neurological disorders. Recently, target-specific genetic manipulations are made possible to perform in combination with specific Cre-lines, albeit costly, labor-intensive and time consuming. Thus, alternative methods of gene manipulations to address important biological questions are highly desirable. In this study, we utilized in utero electroporation technique which involves efficient delivery of hindbrain-specific enhancer/promoter construct, Krox20 into the third ventricle of live mouse embryo to investigate green fluorescent protein (GFP) expression pattern in mouse auditory brainstem and other hindbrain neurons. Results: We created a GFP/DNA construct containing a Krox20 B enhancer and β-globin promoter to drive GFP expression in the hindbrain via injection into the third ventricle of E12 to E13.5 mice. Electrical currents were applied directly to the embryonic hindbrain to allow DNA uptake into the cell. Confocal images were then acquired from fixed brain slices to analyze GFP expression in mouse whole brain at different postnatal stages (P6-P21). By using a cell-type specific enhancer as well as region specific injection and electroporation, robust GFP expression in the cerebellum and auditory brainstem but not in the forebrain was observed. -

Electroporation-Enhanced Gene Delivery in Mammary Tumors
Gene Therapy (2000) 7, 541–547 2000 Macmillan Publishers Ltd All rights reserved 0969-7128/00 $15.00 www.nature.com/gt NONVIRAL TRANSFER TECHNOLOGY RESEARCH ARTICLE Electroporation-enhanced gene delivery in mammary tumors JM Wells, LH Li, A Sen, GP Jahreis and SW Hui Membrane Biophysics Laboratory, Molecular and Cellular Biophysics Department, Roswell Park Cancer Institute, Buffalo, NY 14263-0001, USA Electroporation was applied to enhance gene transfer into pulses 1 ms long were applied across tumors, using caliper subcutaneous MC2 murine breast tumors. Cultured MC2 electrodes on the skin surface. Electric field strengths cells were also transfected by electroporation or by cationic ranged from 400–2300 V/cm. Luciferase expression was liposomes in the presence of serum using pSV-luc plasmids. approximately two orders of magnitude higher than controls Electroporation parameters and liposome formulation were in tumors treated with pulses у800 V/cm. Differences optimized to achieve the highest relative levels of transfec- between enhanced relative levels of transfection using tion. An electric field threshold for successful electrotransfec- uncomplexed plasmid and lipoplexes were not statistically tion in cultured cells appeared around 800–900 V/cm. The significant. Distribution of DNA into tumor tissues was moni- liposomes used contained the cationic lipid dioleoyl-3-trime- tored by fluorescence in situ PCR. The highest numbers of thylammonium propane (DOTAP). Multilamellar vesicles fluorescent cells were found in tumors electroporated follow- (MLV) had a 10-fold advantage over small unilamellar ves- ing the injection of plasmid. The significant transfection icles (SUV) in cell culture transfection. For in vivo gene deliv- improvement shows that in vivo electroporation is a powerful ery, the plasmids were injected either alone, or in complex tool for local gene delivery to tumors. -

Non-Viral in Vitro Gene Delivery: It Is Now Time to Set the Bar!
pharmaceutics Review Non-Viral in Vitro Gene Delivery: It is Now Time to Set the Bar! 1, 1,2, 2 1, Nina Bono y , Federica Ponti y, Diego Mantovani and Gabriele Candiani * 1 GenT Lab, Department of Chemistry, Materials and Chemical Engineering “G. Natta”, Politecnico di Milano, 20131 Milan, Italy; [email protected] (N.B.); [email protected] (F.P.) 2 Laboratory for Biomaterials and Bioengineering, Canada Research Chair I in Biomaterials and Bioengineering for the Innovation in Surgery, Department of Min-Met-Materials Engineering & Research Center of CHU de Quebec, Division of Regenerative Medicine, Laval University, Quebec City, QC G1V 0A6, Canada; [email protected] * Correspondence: [email protected]; Tel.: +39-02-2399-3181 These authors equally contributed to this work. y Received: 3 February 2020; Accepted: 19 February 2020; Published: 21 February 2020 Abstract: Transfection by means of non-viral gene delivery vectors is the cornerstone of modern gene delivery. Despite the resources poured into the development of ever more effective transfectants, improvement is still slow and limited. Of note, the performance of any gene delivery vector in vitro is strictly dependent on several experimental conditions specific to each laboratory. The lack of standard tests has thus largely contributed to the flood of inconsistent data underpinning the reproducibility crisis. A way researchers seek to address this issue is by gauging the effectiveness of newly synthesized gene delivery vectors with respect to benchmarks of seemingly well-known behavior. However, the performance of such reference molecules is also affected by the testing conditions. This survey points to non-standardized transfection settings and limited information on variables deemed relevant in this context as the major cause of such misalignments. -

REVIEW Viral Vectors for Gene Delivery and Gene Therapy Within the Endocrine System
103 REVIEW Viral vectors for gene delivery and gene therapy within the endocrine system D Stone, A David, F Bolognani, P R Lowenstein and M G Castro Molecular Medicine and Gene Therapy Unit, Room 1.302, Stopford Building, School of Medicine, University of Manchester, Oxford Road, Manchester M13 9PT, UK (Requests for offprints should be addressed to M G Castro; Email: [email protected]) (F Bolognani is now at INIBIOLP-Histology B, Faculty of Medicine, National University of La Plata, Argentina) Abstract The transfer of genetic material into endocrine cells and use in gene transfer and gene therapy applications. As differ- tissues, both in vitro and in vivo, has been identified as critical ent viral vector systems have their own unique advantages for the study of endocrine mechanisms and the future treat- and disadvantages, they each have applications for which ment of endocrine disorders. Classical methods of gene they are best suited. This review will discuss viral vector transfer, such as transfection, are inefficient and limited systems that have been used for gene transfer into the mainly to delivery into actively proliferating cells in vitro. endocrine system, and recent developments in viral vector The development of viral vector gene delivery systems is technology that may improve their use for endocrine beginning to circumvent these initial setbacks. Several kinds applications – chimeric vectors, viral vector targeting and of viruses, including retrovirus, adenovirus, adeno-associated transcriptional regulation of transgene expression. virus, and herpes simplex virus, have been manipulated for Journal of Endocrinology (2000) 164, 103–118 Introduction ing of transgenes within mammalian cells is an area of rapid development. -

Receptor-Mediated Gene Delivery to Human Peripheral Blood Mononuclear Cells Using Anti-CD3 Antibody Coupled to Polyethylenimine
Gene Therapy (2001) 8, 362–368 2001 Nature Publishing Group All rights reserved 0969-7128/01 $15.00 www.nature.com/gt RESEARCH ARTICLE Receptor-mediated gene delivery to human peripheral blood mononuclear cells using anti-CD3 antibody coupled to polyethylenimine MM O’Neill, CA Kennedy, RW Barton and RJ Tatake Boehringer Ingelheim Pharmaceuticals, Inc., 900 Ridgebury Road, Ridgefield, CT 06877, USA Gene transfer to primary cells, especially to lymphoid cells, expression was detected until 96 h with a gradual diminution using a nonviral delivery system has been very challenging. in the signal after 48 h. Receptor-mediated gene delivery In the present studies, we have used a cationic polymer, was successfully used for freshly isolated, as well as pre- polyethylenimine (PEI) coupled to an anti-CD3 antibody for viously frozen lymphocyte samples. The transfections per- achieving receptor-mediated gene delivery to human periph- formed using ligands other than anti-CD3 were not as eral blood mononuclear cells (PBMC). Naive, unstimulated efficient as anti-CD3-PEI. These results suggest that in PBMC did not express transfected genes, whereas the addition to receptor-mediated endocytosis, signaling sub- transgenes were expressed efficiently in PHA activated sequent to engagement of the CD3 receptor with anti-CD3- PBMC. Transiently expressed gene products were detected PEI appears to be important for the efficacy of anti-CD3-PEI maximally at 24 and 48 h following transfection. Gene mediated gene delivery. Gene Therapy (2001) 8, 362–368. Keywords: gene delivery; nonviral; receptor-mediated; lymphocytes Introduction providing a co-stimulatory signal has increased the efficiency of retroviral transduction of lymphoid cells.9 Peripheral blood mononuclear cells (PBMC), consist of Pseudotyped retroviruses have also been used in order subpopulations of cells that are involved in specialized to increase the efficiency to transduce lymphoid cells.10–13 effector and regulatory functions in response to immuno- In these studies envelope protein of Moloney murine leu- logical stimuli. -

Gene Delivery
Gene Delivery • Programme: B.Sc (H) Botany • Course Title: Plant Biotechnology • Course code: BOTY 3014 • Prof. Shahana Majumder • Department of Botany • Mahatma Gandhi Central University, Motihari Gene delivery • The gene delivery methods can be divided into two categories, viral and non-viral. • Virus mediated gene delivery utilizes ability of a virus to inject its DNA inside a host cell. A gene that is intended for delivery is packaged into a viral particle. • Non-viral methods include i)physical methods such as microinjection, gene gun, electroporation, by using liposomes and ii) in plants using agrobacterium. Virus mediated • This techniques usually utilize viral vectors – Virus = carrier of desired gene – Virus is usually “crippled” to disable its ability to cause disease – Viral methods have proved to be the most efficient to date – Many viral vectors can stable integrate the desired gene into the target cell’s genome – Problem: Replication defective viruses adversely affect the virus’ normal ability to spread genes in the body Viral vectors Compared to naked DNA, virus particles provide a relatively efficient means of transporting DNA into cells, for expression in the nucleus as recombinant genes (example = adenovirus). [figure from Flint et al. Principles of Virology, ASM Press, 2000] Viral vectors • Retroviruses – eg Moloney murine leukaemia virus • Adenoviruses • Herpes simplex • Adeno-associated viruses (AAV) Physical methods • These are often called as direct methods as they can place the gene in the receiving host directly without the aid of any other organism. Microprojectile bombardment / Gene gun • The gene gun is part of a method called the biolistic (also known as bioballistic) method, and under certain conditions, DNA (or RNA) become “sticky,” adhering to biologically inert particles such as metal atoms (usually tungsten or gold). -

Guidance for Industry: Human Somatic Cell Therapy and Gene Therapy
Guidance for Industry Guidance for Human Somatic Cell Therapy and Gene Therapy Comments and suggestions regarding this document may be submitted at anytime to Dano B. Murphy, HFM-17, Center for Biologics Evaluation and Research, Food and Drug Administration, 1401 Rockville Pike, Rockville, MD 20852-1448. For questions regarding this document, contact Suzanne L. Epstein, Ph.D., 301-827-0450, FAX 301-827-0449, e-mail “[email protected]”. U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research March 1998 TABLE OF CONTENTS Note: page numbering may vary for documents distributed electronically. OVERVIEW (1998) .........................................................1 I. INTRODUCTION ....................................................3 A. Definitions of Somatic Cell Therapy and Gene Therapy ......................3 B. Types of Therapies ................................................. 3 C. Regulatory Considerations ............................................4 D. General Considerations ...............................................5 II. DEVELOPMENT AND CHARACTERIZATION OF CELL POPULATIONS FOR ADMINISTRATION ..................................................7 A. Collection of Cells ..................................................7 B. Cell Culture Procedures ..............................................8 C. Cell Banking Systems Procedures: Generation and Characterization of Master Cell Banks (MCB), Working Cell Banks (WCB), and Producer Cells ...............9 D. Materials