Biodegradable Polymers for Gene Delivery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

University of Birmingham the Increasing Dynamic, Functional
University of Birmingham The increasing dynamic, functional complexity of bio-interface materials Gomes, Bárbara; Simões, Bárbara Sofia; Mendes, Paula DOI: 10.1038/s41570-018-0120 License: None: All rights reserved Document Version Peer reviewed version Citation for published version (Harvard): Gomes, B, Simões, BS & Mendes, P 2018, 'The increasing dynamic, functional complexity of bio-interface materials', Nature Reviews Chemistry, vol. 2, no. 3, 0120. https://doi.org/10.1038/s41570-018-0120 Link to publication on Research at Birmingham portal Publisher Rights Statement: Checked for eligibility 02/11/2018 Published in Nature Reviews Chemistry https://doi.org/10.1038/s41570-018-0120 General rights Unless a licence is specified above, all rights (including copyright and moral rights) in this document are retained by the authors and/or the copyright holders. The express permission of the copyright holder must be obtained for any use of this material other than for purposes permitted by law. •Users may freely distribute the URL that is used to identify this publication. •Users may download and/or print one copy of the publication from the University of Birmingham research portal for the purpose of private study or non-commercial research. •User may use extracts from the document in line with the concept of ‘fair dealing’ under the Copyright, Designs and Patents Act 1988 (?) •Users may not further distribute the material nor use it for the purposes of commercial gain. Where a licence is displayed above, please note the terms and conditions of the licence govern your use of this document. When citing, please reference the published version. -

REVIEW Gene Therapy
Leukemia (2001) 15, 523–544 2001 Nature Publishing Group All rights reserved 0887-6924/01 $15.00 www.nature.com/leu REVIEW Gene therapy: principles and applications to hematopoietic cells VFI Van Tendeloo1,2, C Van Broeckhoven2 and ZN Berneman1 1Laboratory of Experimental Hematology, University of Antwerp (UIA), Antwerp University Hospital (UZA), Antwerp; and 2Laboratory of Molecular Genetics, University of Antwerp (UIA), Department of Molecular Genetics, Flanders Interuniversity Institute for Biotechnology (VIB), Antwerp, Belgium Ever since the development of technology allowing the transfer Recombinant viral vectors of new genes into eukaryotic cells, the hematopoietic system has been an obvious and desirable target for gene therapy. The last 10 years have witnessed an explosion of interest in this Biological gene transfer methods make use of modified DNA approach to treat human disease, both inherited and acquired, or RNA viruses to infect the cell, thereby introducing and with the initiation of multiple clinical protocols. All gene ther- expressing its genome which contains the gene of interest (= apy strategies have two essential technical requirements. ‘transduction’).1 The most commonly used viral vectors are These are: (1) the efficient introduction of the relevant genetic discussed below. In each case, recombinant viruses have had material into the target cell and (2) the expression of the trans- gene at therapeutic levels. Conceptual and technical hurdles the genes encoding essential replicative and/or packaging pro- involved with these requirements are still the objects of active teins replaced by the gene of interest. Advantages and disad- research. To date, the most widely used and best understood vantages of each recombinant viral vector are summarized in vectors for gene transfer in hematopoietic cells are derived Table 1. -

Nanoparticles and Their Biomedical Applications
Review Volume 11, Issue 1, 2021, 8431 - 8445 https://doi.org/10.33263/BRIAC111.84318445 Nanoparticles and their Biomedical Applications Oluwafemi Adeleke Ojo 1,2,* , Israel Idowu Olayide 2 , Maureen Chidinma Akalabu 2, Basiru Olaitan Ajiboye 2 , Adebola Busola Ojo 3 , Babatunji Emmanuel Oyinloye 2,4 , Murugan Ramalingam 5 1 Department of Biochemistry, Landmark University, Omu-Aran, Nigeria 2 Department of Biochemistry, Afe Babalola University, Ado-Ekiti, Nigeria 3 Department of Biochemistry, Ekiti State University, Ado-Ekiti, Nigeria 4 Biotechnology and Structural Biology (BSB) Group, Department of Biochemistry and Microbiology, University of Zululand, KwaDlangezwa 3886, South Africa 5 Biomaterials and stem cell engineering lab, Centre for Biomaterials, Cellular and Molecular Theranostics, School of Mechanical Engineering, Vellore Institute of Technology (VIT) (Deemed to be University), Vellore 632014, India * Correspondence: [email protected]; Scopus Author ID 56415952100 Received: 30.06.2020; Revised: 24.07.2020; Accepted: 26.07.2020; Published: 28.07.2020 Abstract: Over the years, due to the remarkable functional properties, the nanoparticles have been widely used and being tested in the treatment and diagnosis of diseases such as cancer, diabetes, etc. The green synthesis of these nanoparticles can be achieved by physical, chemical, and biological methods. Nanoparticles biosynthesis is put forth to be advantageous over chemical and physical methods because it is non or minimally toxic, environmentally friendly, and cost-effective. A green biosynthesis is an approach that connects nanotechnology with plants, microorganisms, waste materials, and biomolecules. The biological methods help to eliminate destructive processing situations, via letting the synthesis at biological pH, room temperature, and simultaneously, affordable price. -

Gene Delivery & Expression
GENE DELIVERY & EXPRESSION GENE DELIVERY & EXPRESSION LENTIVIRUS, PIGGYBAC, MINICIRCLES, AND MORE SYSTEMBIO.COM SYSTEMS FOR ANY APPLICATION: LENTIVIRAL TOOLS AND MORE In today’s busy labs, getting experiments to work right the first time is critical—not only do you want results you can trust, you want them fast. Which is why SBI has spent over a decade developing a range of exceptionally high-performing products for gene delivery and expression. From our popular high-titer lentivirus production tools to the latest in AAV-based gene delivery systems and a range of reliable integrating and non- integrating gene expression vectors, SBI delivers high-quality, cutting-edge products and services for mammalian gene delivery and expression. With products and services that have been used in thousands of peer-reviewed papers, SBI is your trusted partner for high-quality research. 04 06 12 14 PRODUCT INTEGRATING NON-INTEGRATING SERVICES SELECTOR VECTOR SYSTEMS VECTOR SYSTEMS Syn2Clone Lentiviral Enhanced Episomal Virus Packaging Vectors PiggyBac Cell Line Construction Transposon Minicircle Technology PhiC31 Integrase AAV PinPoint Targeted Integration Non-integrating Lentiviral Gene Knock-out Gene Knock-in Gene Edit AAVS1 Safe Harbor Site INDELS via NHEJ NN OR HR Donor HR Donor Vector Vector HR Donor Vector GENE INSERTION loxP loxP (with HR vector) Excision of GENE KNOCK-OUT selection cassette (with HR vector) CORRECTED GOI PRODUCT SELECTOR A WEALTH OF OPTIONS FOR GENE DELIVERY AND EXPRESSION Integrating Vectors With integrating vectors, the vector DNA becomes permanently incorporated into the genome, often at multiple copy numbers. Depending on the system, integration can be random or directed to a specific locus. -

Laser Scribing Fabrication of Graphitic Carbon Biosensors for Label-Free Detection of Interleukin-6
nanomaterials Article Laser Scribing Fabrication of Graphitic Carbon Biosensors for Label-Free Detection of Interleukin-6 Pei Shee Tan 1,2,† , Eoghan Vaughan 3,† , Jahidul Islam 3, Niall Burke 1, Daniela Iacopino 3,* and Joanna B. Tierney 1,2 1 Shannon Applied Biotechnology Centre, Munster Technological University, Tralee, V92KA43 Kerry, Ireland; [email protected] (P.S.T.); [email protected] (N.B.); [email protected] (J.B.T.) 2 Department of Biological and Pharmaceutical Sciences, Munster Technological University, Tralee, V92KA43 Kerry, Ireland 3 Tyndall National Institute, University College Cork, Dyke Parade, T12R5CP Cork, Ireland; [email protected] (E.V.); [email protected] (J.I.) * Correspondence: [email protected] † These authors contributed equally. Abstract: Interleukin-6 (IL-6) is an important immuno-modulating cytokine playing a pivotal role in inflammatory processes in disease induction and progression. As IL-6 serves as an important indicator of disease state, it is of paramount importance to develop low cost, fast and sensitive improved methods of detection. Here we present an electrochemical immunosensor platform based on the use of highly porous graphitic carbon electrodes fabricated by direct laser writing of commercial polyimide tapes and chemically modified with capture IL-6 antibodies. The unique porous and 3D morphology, as well as the high density of edge planes of the graphitic carbon electrodes, resulted Citation: Tan, P.S.; Vaughan, E.; in a fast heterogeneous electron transfer (HET) rate, k0 = 0.13 cm/s. The resulting immunosensor Islam, J.; Burke, N.; Iacopino, D.; showed a linear response to log of concentration in the working range of 10 to 500 pg/mL, and Tierney, J.B. -

Delivery Strategies of the CRISPR-Cas9 Gene-Editing System for Therapeutic MARK Applications
Journal of Controlled Release 266 (2017) 17–26 Contents lists available at ScienceDirect Journal of Controlled Release journal homepage: www.elsevier.com/locate/jconrel Review article Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic MARK applications ⁎ Chang Liu, Li Zhang, Hao Liu, Kun Cheng Division of Pharmaceutical Sciences, School of Pharmacy, University of Missouri-Kansas City, Kansas City, MO 64108, United States ARTICLE INFO ABSTRACT Keywords: The CRISPR-Cas9 genome-editing system is a part of the adaptive immune system in archaea and bacteria to CRISPR-Cas9 defend against invasive nucleic acids from phages and plasmids. The single guide RNA (sgRNA) of the system Delivery recognizes its target sequence in the genome, and the Cas9 nuclease of the system acts as a pair of scissors to Gene-editing cleave the double strands of DNA. Since its discovery, CRISPR-Cas9 has become the most robust platform for Gene therapy genome engineering in eukaryotic cells. Recently, the CRISPR-Cas9 system has triggered enormous interest in Non-viral delivery therapeutic applications. CRISPR-Cas9 can be applied to correct disease-causing gene mutations or engineer T Nanoparticle cells for cancer immunotherapy. The first clinical trial using the CRISPR-Cas9 technology was conducted in 2016. Despite the great promise of the CRISPR-Cas9 technology, several challenges remain to be tackled before its successful applications for human patients. The greatest challenge is the safe and efficient delivery of the CRISPR-Cas9 genome-editing system to target cells in human body. In this review, we will introduce the mo- lecular mechanism and different strategies to edit genes using the CRISPR-Cas9 system. -

Cellular Shellization: Surface Engineering Gives Cells an Exterior Review Essays Ben Wang1)Y, Peng Liu1)Y and Ruikang Tang1)2)
Prospects & Overviews Cellular shellization: Surface engineering gives cells an exterior Review essays Ben Wang1)y, Peng Liu1)y and Ruikang Tang1)2)Ã Unlike eggs and diatoms, most single cells in nature do Introduction not have structured shells to provide extensive protec- tion. It is a challenge to artificially confer shell structures Progress in biological science and materials engineering has begun to blur the boundary between living and non-living on living cells to improve their inherent properties and systems. Synthetic biologists can now engineer complex, arti- functions. We discuss four different types of cellular ficial biological systems to help understand natural biological shellizations: man-made hydrogels, sol-gels, polyelectro- phenomena and use in a variety of biomedical applications [1]. lytes, and mineral shells. We also explore potential appli- Advances in directed evolution and membrane biophysics cations, such as cell storage, protection, delivery, and make the synthesis of simple living cells an imaginable therapy. We suggest that shellization could provide goal [2]. Cellular life is the basic unit of a living organism and another means to regulate and functionalize cells. defines the presence of a stable information reservoir con- Specifically, the integration of living cells and non-living nected to the external world by a well-defined boundary [3]. functional shells may be developed as a novel strategy The cell membrane separates the interior of a cell from the to create ‘‘super’’ or intelligent cells. Unlike biological outside environment and must meet specific requirements approaches, this material-based bio-interface regulation such as semi-permeability to permit communication and molecular transport across the border [4]. -

Human Inheritable Genetic Modifications
Human Inheritable Genetic Modifications Assessing Scientific, Ethical, Religious, and Policy Issues Prepared by the American Association for the Advancement of Science Mark S. Frankel Audrey R. Chapman September 2000 http://www.aaas.org/spp/dspp/sfrl/germline/main.htm i This report is the product of a collaboration between the authors and a working group convened to advise the authors, and does not necessarily represent the views of American Association for the Advancement of Science or The Greenwall Foundation, which funded this study. Copyright © 2000 American Association for the Advancement of Science Cover: Designed and created by the Office of Publication Services at the American Association for the Advancement of Science. ii Table of Contents Acknowledgements…………………………………………………v Introduction………………………………………………………….1 Major Findings, Concerns, and Recommendations…………………7 Defining Inheritable Genetic Modific ation……………….………..11 Therapeutic Need…………………………………………………..13 Efficacy of Different Approaches to IGM…………………………15 Safety Issues……………………………………………………….23 Inadvertent Germ Line Modific ation………………………………26 Religious Perspectives……………………………………………..27 Ethical Analysis and Considerations……………………………….32 Ethically Appropriate Applications of IGM: Therapy versus Enhancement.………………………………………………………40 Reproductive Rights………………………………………………..44 Balancing Scientific Freedom and Responsibility…………………45 Oversight…………………………………………………………...46 Conclusion.…………………………………………………………56 Glossary…………………………………………………………….59 Appendix A: AAAS Working Group Members……………………65 -

Roadmap on Semiconductor–Cell Biointerfaces
Physical Biology TOPICAL REVIEW Related content - Progress towards biocompatible Roadmap on semiconductor–cell biointerfaces intracortical microelectrodes for neural interfacing applications Mehdi Jorfi, John L Skousen, Christoph To cite this article: Bozhi Tian et al 2018 Phys. Biol. 15 031002 Weder et al. - Roadmap on neurophotonics Yong Ku Cho, Guoan Zheng, George J Augustine et al. View the article online for updates and enhancements. - Implantable optoelectronic probes for in vivo optogenetics Ege Iseri and Duygu Kuzum Recent citations - Advanced nanostructures for cell membrane poration Apresio K Fajrial and Xiaoyun Ding This content was downloaded from IP address 140.247.113.92 on 11/07/2019 at 17:52 IOP Physical Biology Phys. Biol. 15 (2018) 031002 https://doi.org/10.1088/1478-3975/aa9f34 Phys. Biol. 15 TOPICAL REVIEW 2018 Roadmap on semiconductor–cell biointerfaces 2018 IOP Publishing Ltd RECEIVED © 10 August 2017 Bozhi Tian1 , Shuai Xu2,3, John A Rogers4,5, Stefano Cestellos-Blanco5, Peidong Yang5,6,7,8, REVISED 9 9 10 10 11 11 2 November 2017 João L Carvalho-de-Souza , Francisco Bezanilla , Jia Liu , Zhenan Bao , Martin Hjort , Yuhong Cao , PBHIAT 11 12 13 14 15 16 ACCEPTED FOR PUBLICATION Nicholas Melosh , Guglielmo Lanzani , Fabio Benfenati , Giulia Galli , Francois Gygi , Rylan Kautz , 5 December 2017 Alon A Gorodetsky16,17 , Samuel S Kim18, Timothy K Lu18, Polina Anikeeva19, Michal Cifra20 , 031002 PUBLISHED Ondrej Krivosudský20, Daniel Havelka20 and Yuanwen Jiang1 9 March 2018 1 Department of Chemistry, University of Chicago, -
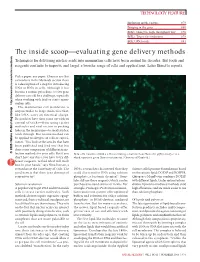
The Inside Scoop—Evaluating Gene Delivery Methods
TECHNOLOGY FEATURE Exploiting mother nature 878 Bringing in the guns 880 BOX 1: Going the high-throughput way 876 BOX 2: Target: the endosome 878 BOX 3: RNAi craze 881 The inside scoop⎯evaluating gene delivery methods Techniques for delivering nucleic acids into mammalian cells have been around for decades. But tools and methods reagents continue to improve and target a broader range of cells and applications. Laura Bonetta reports. Pick a paper, any paper. Chances are that somewhere in the Methods section there .com/nature e is a description of a step for introducing DNA or RNA in cells. Although it has .natur w become a routine procedure, in vitro gene delivery can still be a challenge, especially when working with frail or scarce mam- http://ww malian cells. The mammalian cell membrane is oup impenetrable to large molecules that, r G like DNA, carry an electrical charge. Researchers have thus come up with an arsenal of tricks⎯from using carrier lishing molecules and viral vectors to pocking b holes in the membrane⎯to sneak nucleic Pu acids through. But no one method can be applied to all types of cells or experi- Nature ments. “You look at the articles that have 5 been published and find one that has 200 done some comparison of different trans- © fection methods for your cells. But if you HeLa cells transduced with a self-inactivating retrovirus from Clontech’s pQX (retroQ) series, don’t have any clues, you have to try dif- which expresses green fluorescent protein. (Courtesy of Clontech.) ferent reagents, to find what will work best in your hands,” says Nina Iversen, a researcher at the University of Oslo. -

An Update on the Tools for Creating Transgenic Animal Models of Human Diseases – Focus on Atherosclerosis
Brazilian Journal of Medical and Biological Research (2019) 52(5): e8108, http://dx.doi.org/10.1590/1414-431X20198108 ISSN 1414-431X Review 1/7 An update on the tools for creating transgenic animal models of human diseases – focus on atherosclerosis A.S. Volobueva2, A.N. Orekhov 1,3,4, and A.V. Deykin 1 1Institute of Gene Biology, Russian Academy of Sciences, Moscow, Russia 2Laboratory of Gene Therapy, Biocad Biotechnology Company, Strelnya, Russia 3Laboratory of Angiopathology, Institute of General Pathology and Pathophysiology, Moscow, Russia 4Institute for Atherosclerosis Research, Skolkovo Innovative Center, Moscow, Russia Abstract Animal models of diseases are invaluable tools of modern medicine. More than forty years have passed since the first successful experiments and the spectrum of available models, as well as the list of methods for creating them, have expanded dramatically. The major step forward in creating specific disease models was the development of gene editing techniques, which allowed for targeted modification of the animal’s genome. In this review, we discuss the available tools for creating transgenic animal models, such as transgenesis methods, recombinases, and nucleases, including zinc finger nuclease (ZFN), transcription activator-like effector nuclease (TALEN), and CRISPR/Cas9 systems. We then focus specifically on the models of atherosclerosis, especially mouse models that greatly contributed to improving our understanding of the disease pathogenesis and we outline their characteristics and limitations. Key words: Animal models; Gene editing; Atherosclerosis Introduction Model organisms are widely used in biomedicine reliability of resulting models. Moreover, uncontrolled gene for studying the pathophysiology of human diseases and expression could affect multiple pathways in the animal developing novel therapeutic and diagnostic methods. -

Gene Delivery in Mouse Auditory Brainstem and Hindbrain Using in Utero Electroporation Laurence S David, Jamila Aitoubah, Lee Stephen Lesperance and Lu-Yang Wang*
David et al. Molecular Brain 2014, 7:51 http://www.molecularbrain.com/content/7/1/51 METHODOLOGY Open Access Gene delivery in mouse auditory brainstem and hindbrain using in utero electroporation Laurence S David, Jamila Aitoubah, Lee Stephen Lesperance and Lu-Yang Wang* Abstract Background: Manipulation of gene expression via recombinant viral vectors and creation of transgenic knock-out/ in animals has revolutionized our understanding of genes that play critical roles during neuronal development and pathophysiology of neurological disorders. Recently, target-specific genetic manipulations are made possible to perform in combination with specific Cre-lines, albeit costly, labor-intensive and time consuming. Thus, alternative methods of gene manipulations to address important biological questions are highly desirable. In this study, we utilized in utero electroporation technique which involves efficient delivery of hindbrain-specific enhancer/promoter construct, Krox20 into the third ventricle of live mouse embryo to investigate green fluorescent protein (GFP) expression pattern in mouse auditory brainstem and other hindbrain neurons. Results: We created a GFP/DNA construct containing a Krox20 B enhancer and β-globin promoter to drive GFP expression in the hindbrain via injection into the third ventricle of E12 to E13.5 mice. Electrical currents were applied directly to the embryonic hindbrain to allow DNA uptake into the cell. Confocal images were then acquired from fixed brain slices to analyze GFP expression in mouse whole brain at different postnatal stages (P6-P21). By using a cell-type specific enhancer as well as region specific injection and electroporation, robust GFP expression in the cerebellum and auditory brainstem but not in the forebrain was observed.