Susceptibility of Canadian Flora to EU2 Lineage of Phytophthora Ramorum and Pathogen Sporulation Potential1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
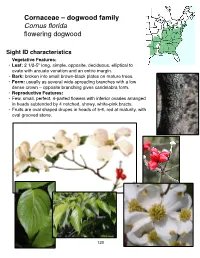
Cornaceae – Dogwood Family Cornus Florida Flowering Dogwood
Cornaceae – dogwood family Cornus florida flowering dogwood Sight ID characteristics Vegetative Features: • Leaf: 2 1/2-5" long, simple, opposite, deciduous, elliptical to ovate with arcuate venation and an entire margin. • Bark: broken into small brown-black plates on mature trees. • Form: usually as several wide-spreading branches with a low dense crown – opposite branching gives candelabra form. • Reproductive Features: • Few, small, perfect, 4-parted flowers with inferior ovaries arranged in heads subtended by 4 notched, showy, white-pink bracts. • Fruits are oval shaped drupes in heads of 5-6, red at maturity, with oval grooved stone. 123 NOTES AND SKETCHES 124 Cornaceae – dogwood family Cornus nuttallii Pacific dogwood Sight ID characteristics Vegetative Features: • Leaf: 2 1/2-4 1/2" long, simple, opposite, deciduous, ovate- elliptical with arcuate venation, margin may be sparsely toothed or entire. • Bark: dark and broken into small plates at maturity. • Form: straight trunk and narrow crown in forested conditions, many-trunked and bushy in open. • Reproductive Features: • Many yellowish-green, small, perfect, 4-parted flowers with inferior ovaries arranged in dense in heads, subtended by 4-7 showy white- pink, petal-like bracts - not notched at the apex. • Fruits are drupes in heads of 30-40, red at maturity and they have smooth stones. 125 NOTES AND SKETCHES 126 Cornaceae – dogwood family Cornus sericea red-osier dogwood Sight ID characteristics Vegetative Features: • Leaf: 2-4" long, simple, opposite, deciduous and somewhat narrow ovate-lanceolate with entire margin. • Twig: bright red, sometimes green splotched with red, white pith. • Bark: red to green with numerous lenticels; later developing larger cracks and splits and turning light brown. -

Likely to Have Habitat Within Iras That ALLOW Road
Item 3a - Sensitive Species National Master List By Region and Species Group Not likely to have habitat within IRAs Not likely to have Federal Likely to have habitat that DO NOT ALLOW habitat within IRAs Candidate within IRAs that DO Likely to have habitat road (re)construction that ALLOW road Forest Service Species Under NOT ALLOW road within IRAs that ALLOW but could be (re)construction but Species Scientific Name Common Name Species Group Region ESA (re)construction? road (re)construction? affected? could be affected? Bufo boreas boreas Boreal Western Toad Amphibian 1 No Yes Yes No No Plethodon vandykei idahoensis Coeur D'Alene Salamander Amphibian 1 No Yes Yes No No Rana pipiens Northern Leopard Frog Amphibian 1 No Yes Yes No No Accipiter gentilis Northern Goshawk Bird 1 No Yes Yes No No Ammodramus bairdii Baird's Sparrow Bird 1 No No Yes No No Anthus spragueii Sprague's Pipit Bird 1 No No Yes No No Centrocercus urophasianus Sage Grouse Bird 1 No Yes Yes No No Cygnus buccinator Trumpeter Swan Bird 1 No Yes Yes No No Falco peregrinus anatum American Peregrine Falcon Bird 1 No Yes Yes No No Gavia immer Common Loon Bird 1 No Yes Yes No No Histrionicus histrionicus Harlequin Duck Bird 1 No Yes Yes No No Lanius ludovicianus Loggerhead Shrike Bird 1 No Yes Yes No No Oreortyx pictus Mountain Quail Bird 1 No Yes Yes No No Otus flammeolus Flammulated Owl Bird 1 No Yes Yes No No Picoides albolarvatus White-Headed Woodpecker Bird 1 No Yes Yes No No Picoides arcticus Black-Backed Woodpecker Bird 1 No Yes Yes No No Speotyto cunicularia Burrowing -

Botanischer Garten Der Universität Tübingen
Botanischer Garten der Universität Tübingen 1974 – 2008 2 System FRANZ OBERWINKLER Emeritus für Spezielle Botanik und Mykologie Ehemaliger Direktor des Botanischen Gartens 2016 2016 zur Erinnerung an LEONHART FUCHS (1501-1566), 450. Todesjahr 40 Jahre Alpenpflanzen-Lehrpfad am Iseler, Oberjoch, ab 1976 20 Jahre Förderkreis Botanischer Garten der Universität Tübingen, ab 1996 für alle, die im Garten gearbeitet und nachgedacht haben 2 Inhalt Vorwort ...................................................................................................................................... 8 Baupläne und Funktionen der Blüten ......................................................................................... 9 Hierarchie der Taxa .................................................................................................................. 13 Systeme der Bedecktsamer, Magnoliophytina ......................................................................... 15 Das System von ANTOINE-LAURENT DE JUSSIEU ................................................................. 16 Das System von AUGUST EICHLER ....................................................................................... 17 Das System von ADOLF ENGLER .......................................................................................... 19 Das System von ARMEN TAKHTAJAN ................................................................................... 21 Das System nach molekularen Phylogenien ........................................................................ 22 -

Cornus Florida
Cornus florida Family: Cornaceae Flowering Dogwood The genus Cornus contains about 40 species which grow in the northern temperate regions of the world. The name cornus is derived from the Latin name of the type species Cornus mas L., Cornelian-cherry of Europe, from the word for horn (cornu), referring to the hardness of the wood. Cornus alternifolia- Alternate Leaf Dogwood, Blue Dogwood, Green-Osier, Pagoda, Pagoda Cornel, Pagoda Dogwood, Pigeonberry, Purple Dogwood, Umbrella-tree Cornus drummondii-Roughleaf Dogwood, Rough-leaved Dogwood Cornus florida- Arrowwood, Boxwood, Bunchberry, Cornel, Dogwood (used bark to treat dog's mange), False Boxwood, Florida Dogwood, Flowering Dogwood, White Cornel Cornus glabrata-Brown Dogwood, Flowering Dogwood, Mountain Dogwood, Pacific Dogwood, Smooth Dogwood, Western Flowering Dogwood Cornus nuttallii-California Dogwood, Flowering Dogwood, Mountain Dogwood, Pacific Dogwood, Western Dogwood, Western Flowering Dogwood Cornus occidentalis-Western Dogwood Cornus racemosa-Blue-fruit Dogwood, Gray Dogwood, Stiffcornel, Stiff Cornel Dogwood, Stiff Dogwood, Swamp Dogwood Cornus rugosa-Roundleaf Dogwood Cornus sessilis-Blackfruit Dogwood, Miners Dogwood Cornus stolonifera-American Dogwood, California Dogwood, Creek Dogwood, Kinnikinnik, Red Dogwood, Red-Osier Dogwood, Red-panicled Dogwood, Redstem Dogwood, Squawbush, Western Dogwood Cornus stricta-Bluefruit Dogwood, Stiffcornel, Stiffcornel Dogwood, Swamp Dogwood The following is for Flowering Dogwood: Distribution North America, from Maine to New York, Ontario, Michigan, Illinois and Missouri south to Kansas, Oklahoma and Texas east to Florida. The Tree Flowering dogwood is well known for its white flower clusters with large white bracts opening in the spring. The fall foliage is bright red. It is a slow growing tree which attains a height of 40 feet and a diameter of 16 inches. -

Cornus Nuttallii 'Monarch'
http://vdberk.demo-account.nl/trees/cornus-nuttallii-monarch/ Cornaceae Cornus Cornus nuttallii 'Monarch' Height 6 - 8 m Crown wide conical , half-open crown, capricious growing Bark and branches red brown to grey, flaking in small plates Leaf wide ovate to oval, green, 6 - 12 cm Attractive autumn colour yellow, orange, red Flowers green yellow in heads, inconspicuous, bracts white, May Fruits ovoid berry-like stone-fruit, Ø 1 cm, bright red Spines/thorns none Toxicity non-toxic (usually) Soil type humus rich content and moisture-retentive Paving tolerates no paving Winter hardiness 7a (-17,7 to -15,0 °C) Wind resistance moderate Light requirement suitable for shadow Fauna tree valuable for butterflies, provides food for birds Application parks, tree containers, theme parks, cemeteries, roof gardens, large gardens, small gardens, patio gardens Type/shape clearstem tree, feathered tree, specimen shrub Origin A. van der Bom, Oudenbosch (NL), before 1970 This cultivar 'Monarch' has an upright habit with a good upright central leader. Therefore it is better suited as a tree, which distinguishes it from the species. Young twigs are green but turn to brown red quickly. Mature trunks too, are red brown to grey. The green leaf turns yellow to orange red in autumn. The flowers are not showy. However, each head with flowers is surrounded by 6 (sometimes 4 or 8) ovoid, pointed bracts. These turn from cream white to entirely white, sometimes with a pink hue and can become 10 cm. This makes the plant in full bloom very decorative. 'Monarch' flowers profusely. The circa 1 cm large, orange-red fruits appear in early autumn. -

Native Plant List CITY of OREGON CITY 320 Warner Milne Road , P.O
Native Plant List CITY OF OREGON CITY 320 Warner Milne Road , P.O. Box 3040, Oregon City, OR 97045 Phone: (503) 657-0891, Fax: (503) 657-7892 Scientific Name Common Name Habitat Type Wetland Riparian Forest Oak F. Slope Thicket Grass Rocky Wood TREES AND ARBORESCENT SHRUBS Abies grandis Grand Fir X X X X Acer circinatumAS Vine Maple X X X Acer macrophyllum Big-Leaf Maple X X Alnus rubra Red Alder X X X Alnus sinuata Sitka Alder X Arbutus menziesii Madrone X Cornus nuttallii Western Flowering XX Dogwood Cornus sericia ssp. sericea Crataegus douglasii var. Black Hawthorn (wetland XX douglasii form) Crataegus suksdorfii Black Hawthorn (upland XXX XX form) Fraxinus latifolia Oregon Ash X X Holodiscus discolor Oceanspray Malus fuscaAS Western Crabapple X X X Pinus ponderosa Ponderosa Pine X X Populus balsamifera ssp. Black Cottonwood X X Trichocarpa Populus tremuloides Quaking Aspen X X Prunus emarginata Bitter Cherry X X X Prunus virginianaAS Common Chokecherry X X X Pseudotsuga menziesii Douglas Fir X X Pyrus (see Malus) Quercus garryana Garry Oak X X X Quercus garryana Oregon White Oak Rhamnus purshiana Cascara X X X Salix fluviatilisAS Columbia River Willow X X Salix geyeriana Geyer Willow X Salix hookerianaAS Piper's Willow X X Salix lucida ssp. lasiandra Pacific Willow X X Salix rigida var. macrogemma Rigid Willow X X Salix scouleriana Scouler Willow X X X Salix sessilifoliaAS Soft-Leafed Willow X X Salix sitchensisAS Sitka Willow X X Salix spp.* Willows Sambucus spp.* Elderberries Spiraea douglasii Douglas's Spiraea Taxus brevifolia Pacific Yew X X X Thuja plicata Western Red Cedar X X X X Tsuga heterophylla Western Hemlock X X X Scientific Name Common Name Habitat Type Wetland Riparian Forest Oak F. -

Seed Collection and Conservation of Cornus Nuttallii (Pacific Dogwood), a Region 1 Sensitive Species
SEED COLLECTION AND CONSERVATION OF CORNUS NUTTALLII (PACIFIC DOGWOOD), A REGION 1 SENSITIVE SPECIES by Christine C. Lorain Natural Heritage Section Nongame/Endangered Wildlife Program Bureau of Wildlife November 1990 Idaho Department of Fish and Game 600 South Walnut, P.O. Box 25 Boise, Idaho 83707 Jerry M. Conley, Director Cooperative Challenge Cost Share Project Clearwater and Nez Perce National Forests Idaho Department of Fish and Game Purchase Order No. 43-0276-0-0317 (CNF) Purchase Order No. 43-0252-0-0293 (NPNF) ABSTRACT Seed of Cornus nuttallii (Pacific dogwood) was collected and preserved from the Clearwater and Nez Perce National Forests during the 1990 field season. This project was conducted by the Idaho Department of Fish and Game's Natural Heritage Program as a cooperative Challenge Cost Share venture between the Department and the Clearwater and Nez Perce National Forests. Pacific dogwood, a Region 1 Sensitive Plant Species, is a Pacific coastal disjunct to northern Idaho. The principal distribution of this species is west of the Cascade/Sierra crest from southwestern British Columbia to southern California. A single isolated population occurs some 300 miles inland along the confluence of the Lochsa and Selway Rivers on lands administered by the Nez Perce and Clearwater National Forests. In the late 1980's, a severe population decline caused by a root disease was noted within the disjunct population. Due to this serious threat, a comprehensive conservation strategy for Pacific dogwood in Idaho was initiated. Part of this strategy concentrated on preserving this unique disjunct population and its potentially valuable (including commercial) gene pool through seed collection and long-term storage. -

Project Budburst Available Species Sheet
Project BudBurst Available Species Sheet www.budburst.org Wildflowers and Herbs Deciduous Trees and Shrubs • Alfalfa (Medicago sativa) • American linden (Tilia americana) • American pasqueflower (Pulsatilla patens aka • Antelope bitterbrush (Purshia tridentata) Anemone patens) • Apple (Malus pumila) • Bigleaf lupine (Lupinus polyphyllus) • Bald cypress (Taxodium distichum) • Bitter root (Lewisia rediviva) • Balsam poplar (Populus balsamifera (aka • California poppy (Eschscholzia californica) trichocarpa)) • Canada thistle (Cirsium arvense) • Beaked hazelnut (Corylus cornuta) • Colorado blue columbine (Aquilegia caerulea) • Bigleaf maple (Acer macrophyllum) • Common dandelion (Taraxacum officinale) • Black elderberry (Sambucus nigra) • Common yarrow (Achillea millefolium) • Black locust (Robinia pseudoacacia) • Darkthroat shootingstar (Dodecatheon • Boxelder (Acer negundo) pulchellum) • Chokecherry (Prunus virginiana) • Dogtooth violet (Erythronium americanum) • Common lilac (Syringa vulgaris) • Field mustard (Brassica rapa) • Common snowberry (Symphoricarpos albus) • Henbit deadnettle (Lamium amplexicaule) • Eastern serviceberry (Amelanchier canadensis) • Indian pink (Spigelia marilandica) • Flowering dogwood (Cornus florida) • Jack in the pulpit (Arisaema triphyllum) • Forsythia (Forsythia xintermedia) • Lanceleaf springbeauty (Claytonia lanceolata) • Lewis' mock orange (Philadelphus lewisii) • Large flowered trillium (Trillium grandiflorum) • Pacific dogwood (Cornus nuttallii) • Mayapple (Podophyllum peltatum) • Paper birch (Betula -

Cornus Nuttallii Western Dogwood, Pacific Dogwood, Mountain Dogwood
http://vdberk.demo-account.nl/trees/cornus-nuttallii/ Cornaceae Cornus Cornus nuttallii Western dogwood, Pacific dogwood, Mountain dogwood Height 6 - 8 m Crown round, sometimes conical, half-open crown, capricious growing Bark and branches red brown to grey, flaking in small plates Leaf wide ovate to oval, green, 6 - 12 cm Attractive autumn colour yellow, orange Flowers green yellow in heads, inconspicuous, bracts white, May Fruits ovate berry-like stone fruits, Ø 1 cm, bright red Spines/thorns none Toxicity non-toxic (usually) Soil type soil rich in humus content and moisture-retentive Paving tolerates no paving Winter hardiness 7a (-17,7 to -15,0 °C) Wind resistance moderate Fauna tree valuable for butterflies, provides food for birds Application parks, tree containers, theme parks, cemeteries, roof gardens, large gardens, small gardens, patio gardens Type/shape clearstem tree, feathered tree, multi-stem tree, specimen tree Origin western part of North America Large upright shrub that is also grown as a tree. It has an upright habit and becomes less wide than other Cornus species. Young twigs are green but turn to brown red rapidly. Mature trunks have a red brown to grey colour. The green leaf turns yellow to orange red in autumn. The flowers are inconspicuous. However, each head with flowers has 6 (sometimes 4 or 8) ovate, pointed bracts. These turn from creamy white to uniformly white with, sometimes, a pink hue. They can get more than 10 cm big. This makes the plant in full bloom very decorative. The circa 1 cm large, orange red fruits appear in early autumn. -

Sensitive Species That Are Not Listed Or Proposed Under the ESA Sorted By: Major Group, Subgroup, NS Sci
Forest Service Sensitive Species that are not listed or proposed under the ESA Sorted by: Major Group, Subgroup, NS Sci. Name; Legend: Page 94 REGION 10 REGION 1 REGION 2 REGION 3 REGION 4 REGION 5 REGION 6 REGION 8 REGION 9 ALTERNATE NATURESERVE PRIMARY MAJOR SUB- U.S. N U.S. 2005 NATURESERVE SCIENTIFIC NAME SCIENTIFIC NAME(S) COMMON NAME GROUP GROUP G RANK RANK ESA C 9 Anahita punctulata Southeastern Wandering Spider Invertebrate Arachnid G4 NNR 9 Apochthonius indianensis A Pseudoscorpion Invertebrate Arachnid G1G2 N1N2 9 Apochthonius paucispinosus Dry Fork Valley Cave Invertebrate Arachnid G1 N1 Pseudoscorpion 9 Erebomaster flavescens A Cave Obligate Harvestman Invertebrate Arachnid G3G4 N3N4 9 Hesperochernes mirabilis Cave Psuedoscorpion Invertebrate Arachnid G5 N5 8 Hypochilus coylei A Cave Spider Invertebrate Arachnid G3? NNR 8 Hypochilus sheari A Lampshade Spider Invertebrate Arachnid G2G3 NNR 9 Kleptochthonius griseomanus An Indiana Cave Pseudoscorpion Invertebrate Arachnid G1 N1 8 Kleptochthonius orpheus Orpheus Cave Pseudoscorpion Invertebrate Arachnid G1 N1 9 Kleptochthonius packardi A Cave Obligate Pseudoscorpion Invertebrate Arachnid G2G3 N2N3 9 Nesticus carteri A Cave Spider Invertebrate Arachnid GNR NNR 8 Nesticus cooperi Lost Nantahala Cave Spider Invertebrate Arachnid G1 N1 8 Nesticus crosbyi A Cave Spider Invertebrate Arachnid G1? NNR 8 Nesticus mimus A Cave Spider Invertebrate Arachnid G2 NNR 8 Nesticus sheari A Cave Spider Invertebrate Arachnid G2? NNR 8 Nesticus silvanus A Cave Spider Invertebrate Arachnid G2? NNR -

Nr 222 Native Tree, Shrub, & Herbaceous Plant
NR 222 NATIVE TREE, SHRUB, & HERBACEOUS PLANT IDENTIFICATION BY RONALD L. ALVES FALL 2014 NR 222 by Ronald L. Alves Note to Students NOTE TO STUDENTS: THIS DOCUMENT IS INCOMPLETE WITH OMISSIONS, ERRORS, AND OTHER ITEMS OF INCOMPETANCY. AS YOU MAKE USE OF IT NOTE THESE TRANSGRESSIONS SO THAT THEY MAY BE CORRECTED AND YOU WILL RECEIVE A CLEAN COPY BY THE END OF TIME OR THE SEMESTER, WHICHEVER COMES FIRST!! THANKING YOU FOR ANY ASSISTANCE THAT YOU MAY GIVE, RON ALVES. Introduction This manual was initially created by Harold Whaley an MJC Agriculture and Natural Resources instruction from 1964 – 1992. The manual was designed as a resource for a native tree and shrub identification course, Natural Resources 222 that was one of the required courses for all forestry and natural resource majors at the college. The course and the supporting manual were aimed almost exclusively for forestry and related majors. In addition to NR 222 being taught by professor Whaley, it has also been taught by Homer Bowen (MJC 19xx -), Marlies Boyd (MJC 199X – present), Richard Nimphius (MJC 1980 – 2006) and currently Ron Alves (MJC 1974 – 2004). Each instructor put their own particular emphasis and style on the course but it was always oriented toward forestry students until 2006. The lack of forestry majors as a result of the Agriculture Department not having a full time forestry instructor to recruit students and articulate with industry has resulted in a transformation of the NR 222 course. The clientele not only includes forestry major, but also landscape designers, environmental horticulture majors, nursery people, environmental science majors, and people interested in transforming their home and business landscapes to a more natural venue. -

Uc Santa Cruz Recommended Native Plants, Non-Native Plants, and Grass
CAMPUS STANDARDS APPENDIX A UC SANTA CRUZ RECOMMENDED NATIVE PLANTS, NON-NATIVE PLANTS, AND GRASS SEED MIXTURES May 1, 1998 5/1/98 UCSC Campus Standards Handbook Plant List Appendix A 1 * May not be appropriate in some campus locations due to messiness or susceptibility to fungus, frost, deer, pests, etc. REDWOOD FORESTS Predominant native species at UCSC with commercial availability Scientific Name Common Name TREES: Acer macrophyllum Bigleaf Maple Sequoia sempervirens Coast Redwood SHRUBS: Corylus cornuta var. californica Western Hazelnut Rosa gymnocarpa Wood Rose Ribes sanguineum var. glutinosum Red Flowering Currant Vaccinium ovatum California Huckleberry GROUND COVER: Oxalis oregana Redwood Sorrell Petasites frigidus var. palmatus Coltsfoot * Polystichum munitum Sword Fern Symphoricarpos mollis Creeeping Snowberry Trillium ovatum Wake Robin Whipplea modesta Yerba De Selva Viola sempervirons Evergreen Violet WET HABITAT GROUNDCOVER: Athyrium filix-femina Lady Fern Equisetum spp. Horse Tail Ranuculus californicus Buttercup Woodwardia fimbriata Giant Chain Fern NATIVE PLANTS ASSOCIATED WITH REDWOOD FORESTS Not necessarily predominant species at UCSC Scientific Name Common Name TREES: Acer macrophyllum Big Leaf Maple Quercus agrifolia Coast Live Oak Torreya californica California Nutmeg Cornus nuttallii Western Dogwood September 2016 UCSC Campus Standards Handbook Plant List Appendix A 2 Quercus chrysolepis Canyon Live Oak Quercus kelloggi California Black Oak Quercus wislizenii Interior Live Oak SHRUBS: Acer circinatum Vine Maple Berberis