Does a Minimal Intervention Approach Threaten the Biodiversity Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Study of the Characteristics of the Appearances of Lepidoptera Larvae and Foodplants at Mt
JOURNAL OF Research Paper ECOLOGY AND ENVIRONMENT http://www.jecoenv.org J. Ecol. Environ. 36(4): 245-254, 2013 A Study of the Characteristics of the Appearances of Lepidoptera Larvae and Foodplants at Mt. Gyeryong National Park in Korea Yong-Gu Han, Sang-Ho Nam, Youngjin Kim, Min-Joo Choi and Youngho Cho* Department of Biology, College of Natural Science, Daejeon University, Daejeon 300-716, Korea Abstract This research was conducted over a time span of three years, from 2009 to 2011. Twenty-one surveys in total, seven times per year, were done between April and June of each year on major trees on trails around Donghaksa and Gapsa in Mt. Gyeryong National Park in order to identify foodplants of the Lepidoptera larvae and their characteristic appearances. During the survey of Lepidoptera larvae in trees along trails around Donghaksa and Gapsa, 377 individuals and 21 spe- cies in 8 families were identified. The 21 species wereAlcis angulifera, Cosmia affinis, Libythea celtis, Adoxophyes orana, Amphipyra monolitha, Acrodontis fumosa, Xylena formosa, Ptycholoma lecheana circumclusana, Choristoneura adum- bratana, Archips capsigeranus, Pandemis cinnamomeana, Rhopobota latipennis, Apochima juglansiaria, Cifuna locuples, Lymantria dispar, Eilema deplana, Rhodinia fugax, Acronicta rumicis, Amphipyra erebina, Favonius saphirinus, and Dra- vira ulupi. Twenty-one Lepidoptera insect species were identified in 21 species of trees, including Zelkova serrata. Among them, A. angulifera, C. affinis, and L. celtis were found to have the widest range of foodplants. Additionally, it was found that many species of Lepidoptera insects can utilize more species as foodplants according to the chemical substances in the plants and environments in addition to the foodplants noted in the literature. -

Xyleninae 73.087 2385 Small Mottled Willow
Xyleninae 73.087 2385 Small Mottled Willow (Spodoptera exigua) 73.089 2386 Mediterranean Brocade (Spodoptera littoralis) 73.091 2396 Rosy Marbled (Elaphria venustula) 73.092 2387 Mottled Rustic (Caradrina morpheus) 73.093 2387a Clancy's Rustic (Caradrina kadenii) 73.095 2389 Pale Mottled Willow (Caradrina clavipalpis) 73.096 2381 Uncertain (Hoplodrina octogenaria) 73.0961 2381x Uncertain/Rustic agg. (Hoplodrina octogenaria/blanda) 73.097 2382 Rustic (Hoplodrina blanda) 73.099 2384 Vine's Rustic (Hoplodrina ambigua) 73.100 2391 Silky Wainscot (Chilodes maritima) 73.101 2380 Treble Lines (Charanyca trigrammica) 73.102 2302 Brown Rustic (Rusina ferruginea) 73.103 2392 Marsh Moth (Athetis pallustris) 73.104 2392a Porter's Rustic (Athetis hospes) 73.105 2301 Bird's Wing (Dypterygia scabriuscula) 73.106 2304 Orache Moth (Trachea atriplicis) 73.107 2300 Old Lady (Mormo maura) 73.109 2303 Straw Underwing (Thalpophila matura) 73.111 2097 Purple Cloud (Actinotia polyodon) 73.113 2306 Angle Shades (Phlogophora meticulosa) 73.114 2305 Small Angle Shades (Euplexia lucipara) 73.118 2367 Haworth's Minor (Celaena haworthii) 73.119 2368 Crescent (Helotropha leucostigma) 73.120 2352 Dusky Sallow (Eremobia ochroleuca) 73.121 2364 Frosted Orange (Gortyna flavago) 73.123 2361 Rosy Rustic (Hydraecia micacea) 73.124 2362 Butterbur (Hydraecia petasitis) 73.126 2358 Saltern Ear (Amphipoea fucosa) 73.127 2357 Large Ear (Amphipoea lucens) 73.128 2360 Ear Moth (Amphipoea oculea) 73.1281 2360x Ear Moth agg. (Amphipoea oculea agg.) 73.131 2353 Flounced Rustic (Luperina -

Noctuid Moth (Lepidoptera, Noctuidae) Communities in Urban Parks of Warsaw
POLISH ACADEMY OF SCIENCES • INSTITUTE OF ZOOLOGY MEMORABILIA ZOOLOGICA MEMORABILIA ZOOL. 42 125-148 1986 GRAŻYNA WINIARSKA NOCTUID MOTH (LEPIDOPTERA, NOCTUIDAE) COMMUNITIES IN URBAN PARKS OF WARSAW ABSTRACT A total of 40 noctuid moth species were recorded in four parks of Warsaw. Respective moth communities consisted of a similar number of species (17—25), but differed in their abundance index (3.5 —7.9). In all the parks, the dominant species were Autographa gamma and Discrestra trifolii. The subdominant species were represented by Acronicta psi, Trachea atriplicis, Mamestra suasa, Mythimna pallens, and Catocala nupta. There were differences in the species composition and dominance structure among noctuid moth communities in urban parks, suburban linden- oak-hornbeam forest, and natural linden-oak-hornbeam forest. In the suburban and natural linden-oak-hornbeam forests, the number of species was higher by 40% and their abundance wao 5 — 9 times higher than in the urban parks. The species predominating in parks occurred in very low numbers in suburban and natural habitats. Only T. atriplicis belonged to the group of most abundant species in all the habitats under study. INTRODUCTION In recent years, the interest of ecologists in urban habitats has been increasing as they proved to be rich in plant and animal species. The vegetation of urban green areas is sufficiently well known since its species composition and spatial structure are shaped by gardening treatment. But the fauna of these areas is poorly known, and regular zoological investigations in urban green areas were started not so long ago, when urban green was recognized as one of the most important factors of the urban “natural” habitat (Ciborowski 1976). -
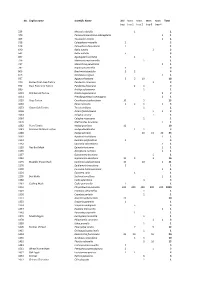
Full List Here
No. English name Scientific Name JBM Mark Mark Mark Mark Total trap trap 1 trap 2 trap 3 trap 4 229 Monopis obviella 1 1 440 Paraswammerdamia albicapitella 1 1 463 Ypsolopha vittella 1 1 518 Coleophora mayrella 2 2 519 Coleophora deauratella 1 1 640 Batia lunaris 2 2 642 Batia unitella 5 5 697 Agonopterix arenella 1 1 726 Metzneria metzneriella 1 1 737 Monochroa palustrella 1 1 787 Bryotropha terrella 3 3 866 Brachmia blandella 2 2 4 873 Blastobasis lignea 1 1 937 Agapeta hamana 3 5 10 18 970 Barred Fruit-tree Tortrix Pandemis cerasana 2 2 972 Dark Fruit-tree Tortrix Pandemis heparana 2 1 3 980 Archips xylosteana 1 1 1010 Red-barred Tortrix Ditula angustiorana 2 2 1011 Pseudargyrotoza conwagana 1 1 1020 Grey Tortrix Cnephasia stephensiana 10 5 15 1030 Eana incanana 1 2 1 1 5 1033 Green Oak Tortrix Tortrix viridana 1 1 1036 Acleris forsskaleana 1 1 2 1063 Celypha striana 1 1 1064 Celypha rosaceana 1 1 1076 Olethreutes lacunana 1 1 1082 Plum Tortrix Hedya pruniana 10 10 1083 Marbled Orchard Tortrix Hedya dimidioalba 1 1 2 1086 Hedya salicella 10 15 20 45 1092 Apotomis turbidana 1 1 1113 Eudemis profundana 5 5 1132 Eponotia subocellana 1 1 1139 Nut Bud Moth Epinotia tenerana 1 1 1165 Zeiraphera isertana 1 1 1167 Gypsonoma aceriana 1 1 1169 Gypsonoma dealbana 10 3 3 16 1175 Bramble Shoot Moth Epiblema uddmanniana 10 10 1 21 1176 Epiblema trimaculana 1 1 1200 Eucosma hohenwartiana 1 1 1201 Eucosma cana 1 1 1205 Bud Moth Spilonota ocellana 1 1 1260 Cydia splendana 3 3 1261 Codling Moth Cydia pomonella 1 1 1293 Chrysoteuchia culmella 200 200 -

Diptera) of the Czech Republic
© Entomologica Fennica. 30 March 2009 Annotated host catalogue for the Tachinidae (Diptera) of the Czech Republic Jaromir Vafihara*, Hans-Peter Tschorsnig, Benno Herting’r, Petr Mfickstein & Veronika Michalkova J P. & V. Vanhara, ., Tschorsnig, H.-P., Herting, B., Miickstein, Michalkova, 2009: Annotated host catalogue for the Tachinidae (Diptera) of the Czech Re- public. — Entomol. Fennica 20: 22—48. An annotated host catalogue is given for the Tachinidae ofthe Czech Republic. It comprises 149 of476 tachinid species which are currently known from this coun- try (included the two new records cited below). 195 hosts are listed. The first host records ofTachinidae date back to the second halfofthe 19th century. The bibli- ography for the host records consists of 1 16 papers of 55 researchers. Several re- cords of hitherto unpublished material are included. Phryxe setifacies and Anthomyiopsis plagioderae are first records for the Czech Republic. J. Vanhara (*corresponding author), Masaryk University, Faculty ofScience, Kotlarska 2, CZ—6I I 3 7 Brno, Czech Republic, [email protected] H.—P. Tschorsnig, Staatliches Museumflir Naturkunde, Rosenstein I, D— 70 191 Stuttgart, Germany, tschorsnig.smns@naturkundemuseum—bw.de P. Muckstein Administration of the Protected Landscape Area Zd’drske' vrchy, Brnenska 39, CZ—591 01 Zd’dr nad Sazavou, Czech Republic, muchstein @email.cz V. Michalkova, Masaryk University, Faculty ofScience, Kotlarska 2, CZ—6I I 3 7 Brno, Czech Republic, [email protected] Received 22 August 200 7, accepted 21 January 2008 1. Introduction The tachinid species are listed in their actual valid nomenclature; probable misidentifications Tachinidae are a very large and important dipter- are — if possible — tentatively corrected, but the an family of (mainly) insect parasitoids. -

The Entomologist's Record and Journal of Variation
M DC, — _ CO ^. E CO iliSNrNVINOSHilWS' S3ldVyan~LIBRARlES*"SMITHS0N!AN~lNSTITUTl0N N' oCO z to Z (/>*Z COZ ^RIES SMITHSONIAN_INSTITUTlON NOIiniIiSNI_NVINOSHllWS S3ldVaan_L: iiiSNi'^NviNOSHiiNS S3iavyan libraries Smithsonian institution N( — > Z r- 2 r" Z 2to LI ^R I ES^'SMITHSONIAN INSTITUTlON'"NOIini!iSNI~NVINOSHilVMS' S3 I b VM 8 11 w </» z z z n g ^^ liiiSNi NviNOSHims S3iyvyan libraries Smithsonian institution N' 2><^ =: to =: t/J t/i </> Z _J Z -I ARIES SMITHSONIAN INSTITUTION NOIiniliSNI NVINOSHilWS SSIdVyan L — — </> — to >'. ± CO uiiSNi NViNosHiiws S3iyvaan libraries Smithsonian institution n CO <fi Z "ZL ~,f. 2 .V ^ oCO 0r Vo^^c>/ - -^^r- - 2 ^ > ^^^^— i ^ > CO z to * z to * z ARIES SMITHSONIAN INSTITUTION NOIinillSNl NVINOSHllWS S3iaVdan L to 2 ^ '^ ^ z "^ O v.- - NiOmst^liS^> Q Z * -J Z I ID DAD I re CH^ITUCnMIAM IMOTtTIITinM / c. — t" — (/) \ Z fj. Nl NVINOSHIIINS S3 I M Vd I 8 H L B R AR I ES, SMITHSONlAN~INSTITUTION NOIlfl :S^SMITHS0NIAN_ INSTITUTION N0liniliSNI__NIVIN0SHillMs'^S3 I 8 VM 8 nf LI B R, ^Jl"!NVINOSHimS^S3iavyan"'LIBRARIES^SMITHS0NIAN~'lNSTITUTI0N^NOIin L '~^' ^ [I ^ d 2 OJ .^ . ° /<SS^ CD /<dSi^ 2 .^^^. ro /l^2l^!^ 2 /<^ > ^'^^ ^ ..... ^ - m x^^osvAVix ^' m S SMITHSONIAN INSTITUTION — NOIlfliliSNrNVINOSHimS^SS iyvyan~LIBR/ S "^ ^ ^ c/> z 2 O _ Xto Iz JI_NVIN0SH1I1/MS^S3 I a Vd a n^LI B RAR I ES'^SMITHSONIAN JNSTITUTION "^NOlin Z -I 2 _j 2 _j S SMITHSONIAN INSTITUTION NOIinillSNI NVINOSHilWS S3iyVaan LI BR/ 2: r- — 2 r- z NVINOSHiltNS ^1 S3 I MVy I 8 n~L B R AR I Es'^SMITHSONIAN'iNSTITUTIOn'^ NOlin ^^^>^ CO z w • z i ^^ > ^ s smithsonian_institution NoiiniiiSNi to NviNosHiiws'^ss I dVH a n^Li br; <n / .* -5^ \^A DO « ^\t PUBLISHED BI-MONTHLY ENTOMOLOGIST'S RECORD AND Journal of Variation Edited by P.A. -

Diversity of the Moth Fauna (Lepidoptera: Heterocera) of a Wetland Forest: a Case Study from Motovun Forest, Istria, Croatia
PERIODICUM BIOLOGORUM UDC 57:61 VOL. 117, No 3, 399–414, 2015 CODEN PDBIAD DOI: 10.18054/pb.2015.117.3.2945 ISSN 0031-5362 original research article Diversity of the moth fauna (Lepidoptera: Heterocera) of a wetland forest: A case study from Motovun forest, Istria, Croatia Abstract TONI KOREN1 KAJA VUKOTIĆ2 Background and Purpose: The Motovun forest located in the Mirna MITJA ČRNE3 river valley, central Istria, Croatia is one of the last lowland floodplain 1 Croatian Herpetological Society – Hyla, forests remaining in the Mediterranean area. Lipovac I. n. 7, 10000 Zagreb Materials and Methods: Between 2011 and 2014 lepidopterological 2 Biodiva – Conservation Biologist Society, research was carried out on 14 sampling sites in the area of Motovun forest. Kettejeva 1, 6000 Koper, Slovenia The moth fauna was surveyed using standard light traps tents. 3 Biodiva – Conservation Biologist Society, Results and Conclusions: Altogether 403 moth species were recorded Kettejeva 1, 6000 Koper, Slovenia in the area, of which 65 can be considered at least partially hygrophilous. These results list the Motovun forest as one of the best surveyed regions in Correspondence: Toni Koren Croatia in respect of the moth fauna. The current study is the first of its kind [email protected] for the area and an important contribution to the knowledge of moth fauna of the Istria region, and also for Croatia in general. Key words: floodplain forest, wetland moth species INTRODUCTION uring the past 150 years, over 300 papers concerning the moths Dand butterflies of Croatia have been published (e.g. 1, 2, 3, 4, 5, 6, 7, 8). -

Checklist of Cumbrian Lepidoptera (2000)
A Checklist of the Butterflies and larger Moths of Cumbria Edited by Bill Kydd and Stephen Hewitt A Checklist of the Butterflies and larger Moths of Cumbria Edited by Bill Kydd and Stephen Hewitt February 2000 Tullie House Museum and Art Gallery Castle Street Carlisle © Carlisle City Council 2000 The Cumbria Biological Records Database at Tullie House Museum Tullie House Museum maintains a database of wildlife sightings from across the county of Cumbria. Some 140,000 records from Cumbria are stored on computer, backed up by large collections of voucher and reference specimens. The Museum aims to record and monitor the status of wildlife in Cumbria. The information is used to increase the knowledge of the wildlife of the county and to inform decisions affecting the wildlife and countryside of Cumbria. Tullie House welcomes information and records concerning the wildlife of Cumbria. Provisional distribution atlases are available for the following groups: Mammals in Cumbria 1999 Butterflies in Cumbria 2000 Dragonflies in Cumbria 1994 Grasshoppers and Crickets in Cumbria 1993 Ladybirds in Cumbria 1998 Reptiles in Cumbria 1992 Amphibians in Cumbria 1995 Please send your wildlife sightings to the Keeper of Natural Sciences, Tullie House Museum, Castle Street, Carlisle CA3 8TP. Tel: 01228 534781 ext. 248. Fax: 01228 810249. E-mail: [email protected] Introduction The purpose of this checklist is to assist local moth recorders in assessing their own records and captures. We hope that it will enable recorders to identify unusual and important records as they occur and further stimulate the study of moths in Cumbria. Bill Kydd acted as County Lepidoptera Recorder for Cumbria Naturalists’ Union from 1979 to 1997. -

Planting of Disease-Resistant Elm Cultivars for Lepidoptera and the Biosecurity Implications
Planting of Disease-resistant Elm Cultivars for Lepidoptera and the Biosecurity Implications Policy Summary Butterfly Conservation supports the planting of disease-resistant elm (DRE) under specific circumstances and when strict biosecurity measures are implemented, as part of a wider strategy to conserve native and naturalised elm. Butterfly Conservation will preferentially use UK sourced and grown trees and would support nurseries to propagate native, naturalised and DREs within the UK. Biosecurity is the primary consideration when procuring trees. Conserving and enhancing native and naturalised elm in rural and urban areas should be an integral part of any elm management strategy. Areas that are considered suitable and unsuitable for native, naturalised and DRE planting are listed in Table 1. Plant native and naturalised elm derived from local provenance as part of the elm species mix if there is no natural seed source or if native and naturalised elm is not present in a range of different age classes. Planting DREs should be undertaken only where locally appropriate whilst meeting strict biosecurity measures. DRE planting sites should be chosen strategically to provide connectivity between colonies and act as refuges, should Dutch Elm Disease (DED) occur, for Section 41 and Section 7 species of Lepidoptera such as the White-letter Hairstreak Satyrium w-album and White-spotted Pinion Cosmia affinis (NERC, 2006; Environmental (Wales) Act 2016). Planted elms should be monitored for breeding White-letter Hairstreak and White-spotted Pinion if within or near the species current distribution (Appendix 1-2). Background Information The outbreak of DED in the UK during the 1970s led to the loss of mature elms throughout the UK. -

Invertebrate Survey Report
Appendix A Invertebrate Survey Report INVERTEBRATE SURVEY OF NORTHSTOWE, CAMBRIDGESHIRE MARK G. TELFER 16TH SEPTEMBER 2013 VERSION 2: 18TH OCTOBER 2013 THIS REPORT WAS PRODUCED FOR OVE ARUP & PARTNERS LTD. Dr. Mark G. Telfer 10, Northall Road Eaton Bray DUNSTABLE LU6 2DQ [email protected] http://markgtelfer.co.uk/ This report should be quoted as: Telfer, M.G. (2013). Invertebrate survey of Northstowe, Cambridgeshire. Version 2. Report for Ove Arup and Partners Ltd. Invertebrate survey of Northstowe Contents 1 SUMMARY .......................................................................................................................................... 4 2 INTRODUCTION ................................................................................................................................... 4 2.1 BACKGROUND .............................................................................................................................................. 4 2.2 ECOLOGY ..................................................................................................................................................... 5 2.3 LEGISLATION AND CONSERVATION STATUS ......................................................................................................... 5 2.4 OBJECTIVES .................................................................................................................................................. 6 3 METHODS .......................................................................................................................................... -

The Lepidoptera of Bucharest and Its Surroundings (Romania)
Travaux du Muséum National d’Histoire Naturelle © 30 Décembre Vol. LIV (2) pp. 461–512 «Grigore Antipa» 2011 DOI: 10.2478/v10191-011-0028-9 THE LEPIDOPTERA OF BUCHAREST AND ITS SURROUNDINGS (ROMANIA) LEVENTE SZÉKELY Abstract. This study presents a synthesis of the current knowledge regarding the Lepidoptera fauna of Bucharest and the surrounding areas within a distance up to 50 kilometers around the Romanian capital. Data about the fauna composition are presented: the results of the research work beginning with the end of the 19th century, as well the results of the research work carried out in the last 15 years. The research initiated and done by the author himself, led to the identification of 180 species which were unknown in the past. Even if the natural habitats from this region have undergone through radical changes in the 20th century, the area still preserves a quite rich and interesting Lepidoptera fauna. The forests provide shelter to rich populations of the hawk moth Dolbina elegans A. Bang-Haas, 1912, one of the rarest Sphingidae in Europe, and some other species with high faunistical and zoogeographical value as: Noctua haywardi (Tams, 1926) (it is new record for the Romanian fauna from this area), Catocala dilecta (Hübner, 1808), Tarachidia candefacta (Hübner, [1831]), Chrysodeixis chalcites (Esper, [1789]), Aedia leucomelas (Linnaeus, 1758), and Hecatera cappa (Hübner, [1809]). We also present and discuss the current status of the protected Lepidoptera species from the surroundings of the Romanian capital for the first time. Résumé. Ce travail représente une synthèse des connaissances actuelles concernant la faune de lépidoptères de Bucarest et de ses zones limitrophes sur un rayon de 50 km autour de la capitale de la Roumanie. -
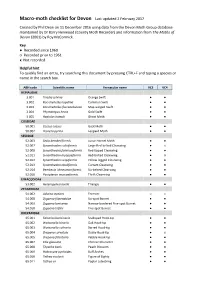
Macro-Moth Checklist for Devon Last Updated 2 February 2017
Macro-moth checklist for Devon Last updated 2 February 2017 Created by Phil Dean on 11 December 2016 using data from the Devon Moth Group database maintained by Dr Barry Henwood (County Moth Recorder) and information from The Moths of Devon (2001) by Roy McCormick. Key ● Recorded since 1960 ○ Recorded prior to 1961 x Not recorded Helpful hint To quickly find an entry, try searching this document by pressing CTRL+F and typing a species or name in the search box. ABH code Scientific name Vernacular name VC3 VC4 HEPIALIDAE 3.001 Triodia sylvina Orange Swift ● ● 3.002 Korscheltellus lupulina Common Swift ● ● 3.003 Korscheltellus fusconebulosa Map-winged Swift ● ● 3.004 Phymatopus hecta Gold Swift ● ● 3.005 Hepialus humuli Ghost Moth ● ● COSSIDAE 50.001 Cossus cossus Goat Moth ● ● 50.002 Zeuzera pyrina Leopard Moth ● ● SESIIDAE 52.003 Sesia bembeciformis Lunar Hornet Moth ● ● 52.007 Synanthedon culiciformis Large Red-belted Clearwing ● x 52.008 Synanthedon formicaeformis Red-tipped Clearwing ● ● 52.011 Synanthedon myopaeformis Red-belted Clearwing ● ○ 52.012 Synanthedon vespiformis Yellow-legged Clearwing ● ● 52.013 Synanthedon tipuliformis Currant Clearwing ● ● 52.014 Bembecia ichneumoniformis Six-belted Clearwing ● ● 52.016 Pyropteron muscaeformis Thrift Clearwing ● ● LIMACODIDAE 53.002 Heterogenea asella Triangle ● ● ZYGAENIDAE 54.002 Adscita statices Forester ○ x 54.008 Zygaena filipendulae Six-spot Burnet ● ● 54.009 Zygaena lonicerae Narrow-bordered Five-spot Burnet ● ● 54.010 Zygaena trifolii Five-spot Burnet ● ● DREPANIDAE 65.001 Falcaria