Banana Moth on Palms, Hodel and Santos, 2020-12
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The New Age of Extinction
Wednesday, Apr. 01, 2009 The New Age of Extinction By Bryan Walsh / Madagascar There are at least 8 million unique species of life on the planet, if not far more, and you could be forgiven for believing that all of them can be found in Andasibe. Walking through this rain forest in Madagascar is like stepping into the library of life. Sunlight seeps through the silky fringes of the Ravenea louvelii, an endangered palm found, like so much else on this African island, nowhere else. Leaf-tailed geckos cling to the trees, cloaked in green. A fat Parson's chameleon lies lazily on a branch, beady eyes scanning for dinner. But the animal I most hoped to find, I don't see at first; I hear it, though — a sustained groan that electrifies the forest quiet. My Malagasy guide, Marie Razafindrasolo, finds the source of the sound perched on a branch. It is the black-and-white indri, largest of the lemurs — a type of small primate found only in Madagascar. The cry is known as a spacing call, a warning to other indris to keep their distance, to prevent competition for food. But there's not much risk of interlopers. The species — like many other lemurs, like many other animals in Madagascar, like so much of life on Earth — is endangered and dwindling fast. Madagascar — which separated from India 80 million to 100 million years ago before eventually settling off the southeastern coast of Africa — is in many ways an Earth apart. All that time in geographic isolation made Madagascar a Darwinian playground, its animals and plants evolving into forms utterly original. -
![Syagrus Romanzoffiana [Cham.] Glassman](https://docslib.b-cdn.net/cover/5715/syagrus-romanzoffiana-cham-glassman-115715.webp)
Syagrus Romanzoffiana [Cham.] Glassman
SCIENTIFIC note Doi: https://doi.org/10.17584/rcch.2019v13i3.8363 Pre-depulping and depulping treatments and the emergence of queen palm seeds (Syagrus romanzoffiana [Cham.] Glassman) Tratamiento de pre-despulpado y despulpado sobre la emergencia de semillas de palma reina (Syagrus romanzoffiana [Cham.] Glassman) LUCAS MARQUEZAN NASCIMENTO1 EDUARDO PRADI VENDRUSCOLO2, 4 LUIZ FERNANDES CARDOSO CAMPOS1 LISMAÍRA GONÇALVES CAIXETA GARCIA1 LARISSA LEANDRO PIRES1 ALEXANDER SELEGUINI3 Syagrus romanzoffiana under conditions of Brazilian Cerrado. Photo: L.M. Nascimento ABSTRACT The propagation of the palm Syagrus romanzoffiano is done sexually with seeds, making the process of obtai- ning new plants slow and difficult, especially on large scales. In addition, seed germination is slow, uneven and susceptible to degradation and loss of vigor because of embryo deterioration, even under laboratory conditions. As a result of the lack of information on efficient depulping methods for queen palm fruits, the present study aimed to establish a depulping methodology that is less aggressive to embryos, maintaining emergence quality. This experiment was carried out in Goiânia, Brazil, using fruits from eight stock plants submitted to three pre-depulping treatments (control, fermentation and drying) and two depulping me- thods (industrial depulping and concrete-mixer with the addition of gravel). After the different pre-sowing processes, the fresh and dry pyrenes mass, remaining fibers adhered to the pyrene and seedling emergence were evaluated. The pulper removed an average of 45% more pyrene pulp than the concrete mixer. However, these methodologies did not result in differences in the emergence of plants, which was affected only by the pre-depulping treatment, with superiority in the use of fresh fruits. -
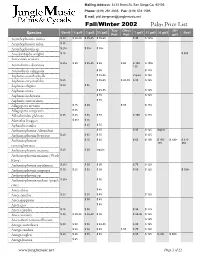
Winter-Fall Sale 2002 Palm Trees-Web
Mailing Address: 3233 Brant St. San Diego Ca, 92103 Phone: (619) 291 4605 Fax: (619) 574 1595 E mail: [email protected] Fall/Winter 2002 Palm Price List Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Acanthophoenix crinita $ 30 $ 30-40 $ 35-45 $ 55-65 $ 95 $ 125+ Acanthophoenix rubra $ 35 Acanthophoenix sp. $ 25+ $ 35+ $ 55+ Acoelorrhaphe wrightii $ 15 $ 300 Acrocomia aculeata $ 25+ $ 35 $ 35-45 $ 65 $ 65 $ 100- $ 150+ Actinokentia divaricata 135 Actinorhytis calapparia $ 55 $ 125 Aiphanes acanthophylla $ 45-55 inquire $ 125 Aiphanes caryotaefolia $ 25 $ 55-65 $ 45-55 $ 85 $ 125 Aiphanes elegans $ 20 $ 35 Aiphanes erosa $ 45-55 $ 125 Aiphanes lindeniana $ 55 $ 125 Aiphanes vincentsiana $ 55 Allagoptera arenaria $ 25 $ 40 $ 55 $ 135 Allagoptera campestris $ 35 Alloschmidtia glabrata $ 35 $ 45 $ 55 $ 85 $ 150 $ 175 Alsmithia longipes $ 35+ $ 55 Aphandra natalia $ 35 $ 55 Archontophoenix Alexandrae $ 55 $ 85 $ 125 inquire Archontophoenix Beatricae $ 20 $ 35 $ 55 $ 125 Archontophoenix $ 25 $ 45 $ 65 $ 100 $ 150- $ 200+ $ 310- 175 350 cunninghamiana Archontophoenix maxima $ 25 $ 30 inquire Archontophoenix maxima (Wash River) Archontophoenix myolaensis $ 25+ $ 30 $ 50 $ 75 $ 125 Archontophoenix purpurea $ 30 $ 25 $ 35 $ 50 $ 85 $ 125 $ 300+ Archontophoenix sp. Archontophoenix tuckerii (peach $ 25+ $ 55 river) Areca alicae $ 45 Areca catechu $ 20 $ 35 $ 45 $ 125 Areca guppyana $ 30 $ 45 Areca ipot $ 45 Areca triandra $ 25 $ 30 $ 95 $ 125 Areca vestiaria $ 25 $ 30-35 $ 35-40 $ 55 $ 85-95 $ 125 Arecastrum romanzoffianum $ 125 Arenga australasica $ 20 $ 30 $ 35 $ 45-55 $ 85 $ 125 Arenga caudata $ 20 $ 30 $ 45 $ 55 $ 75 $ 100 Arenga engleri $ 20 $ 60 $ 35 $ 45 $ 85 $ 125 $ 200 $ 300+ Arenga hastata $ 25 www.junglemusic.net Page 1 of 22 Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Arenga hookeriana inquire Arenga micranthe 'Lhutan' $ 20 inquire Arenga pinnata $ 35 $ 50 $ 85 $ 125 Arenga sp. -

Trachycarpus Fortunei) and Its Application Potential
Article Anatomy of the Windmill Palm (Trachycarpus fortunei) and Its Application Potential Jiawei Zhu 1, Jing Li 1,2, Chuangui Wang 3 and Hankun Wang 1,* 1 Department of Biomaterials, International Center for Bamboo and Rattan, State Forestry Administration and Beijing Co-Built Key Lab for Bamboo and Rattan Science & Technology, Beijing 100102, China; [email protected] (J.Z.); [email protected] (J.L.) 2 Key Lab of Wood Science and Technology of National Forestry and Grassland Administration, Research Institute of Wood Industry, Chinese Academy of Forestry, Beijing 100102, China 3 School of Forestry & Landscape Architecture, Anhui Agricultural University, Hefei 230036, China; [email protected] * Correspondence: [email protected] Received: 13 November 2019; Accepted: 9 December 2019; Published: 10 December 2019 Abstract: The windmill palm (Trachycarpus fortunei (Hook.) H. Wendl.) is widely distributed and is an important potential source of lignocellulosic materials. The lack of knowledge on the anatomy of the windmill palm has led to its inefficient use. In this paper, the diversity in vascular bundle types, shape, surface, and tissue proportions in the leaf sheaths and stems were studied with digital microscopy and scanning electron microscope (SEM). Simultaneously, fiber dimensions, fiber surfaces, cell wall ultrastructure, and micromechanics were studied with atomic force microscopy (AFM) and a nanoindenter. There is diversity among vascular bundles in stems and leaf sheaths. All vascular bundles in the stems are type B (circular vascular tissue (VT) at the edge of the fibrous sheath (FS)) while the leaf sheath vascular bundles mostly belong to type C (aliform (VT) at the center of the (FS), with the wings of the (VT) extending to the edge of the vascular bundles). -

Coconut and Other Palm Trees Posted on August 8, 2019 by Leslie Lang
HOME HOURS & DIRECTIONS GARDEN SLIDESHOW GARDEN NEWS & BLOG Coconut and Other Palm Trees Posted on August 8, 2019 by Leslie Lang Of all the types of palm trees, many people here in Hawai‘i are most familiar with the coconut palm, Cocos nucifera. It’s the tree that says, “tropics.” But there’s so much more to the coconut palm. Its fruit, the niu or coconut, is so useful that early Polynesians brought it along to sustain themselves when they sailed across the Pacific to Hawai‘i. Polynesians knew that when they settled on new islands, they could plant coconuts and make use of the entire tree that grew—not only the coconut meat and water, but also the leaves, the wood, the fiber, and every other part. According to the book Canoe Plants of Ancient Hawaii, “Besides drink, food and shade, niu offers the possibilities of housing, thatching, hats, baskets, furniture, mats, cordage, clothing, charcoal, brooms, fans, ornaments, musical instruments, shampoo, containers, implements and oil for fuel, light, ointments, soap and more.” The only palm tree that’s native to Hawai‘i is the loulu (Pritchardia). There are perhaps 19 loulu species in Hawai‘i and a few related species in Tahiti and Fiji. Hawai‘i used to have large loulu forests, but while some loulu still survive in the wild, many disappeared because of rats, pigs, goats, and even people. Within the genus Pritchardia, there are 25 species of palms native to the tropical Pacific Islands. In Hawai‘i, as many as 19 species of Pritchardia are endemic, and some of them are categorized as endangered, rare, or vulnerable. -

Report-VIC-Croajingolong National Park-Appendix A
Croajingolong National Park, Victoria, 2016 Appendix A: Fauna species lists Family Species Common name Mammals Acrobatidae Acrobates pygmaeus Feathertail Glider Balaenopteriae Megaptera novaeangliae # ~ Humpback Whale Burramyidae Cercartetus nanus ~ Eastern Pygmy Possum Canidae Vulpes vulpes ^ Fox Cervidae Cervus unicolor ^ Sambar Deer Dasyuridae Antechinus agilis Agile Antechinus Dasyuridae Antechinus mimetes Dusky Antechinus Dasyuridae Sminthopsis leucopus White-footed Dunnart Felidae Felis catus ^ Cat Leporidae Oryctolagus cuniculus ^ Rabbit Macropodidae Macropus giganteus Eastern Grey Kangaroo Macropodidae Macropus rufogriseus Red Necked Wallaby Macropodidae Wallabia bicolor Swamp Wallaby Miniopteridae Miniopterus schreibersii oceanensis ~ Eastern Bent-wing Bat Muridae Hydromys chrysogaster Water Rat Muridae Mus musculus ^ House Mouse Muridae Rattus fuscipes Bush Rat Muridae Rattus lutreolus Swamp Rat Otariidae Arctocephalus pusillus doriferus ~ Australian Fur-seal Otariidae Arctocephalus forsteri ~ New Zealand Fur Seal Peramelidae Isoodon obesulus Southern Brown Bandicoot Peramelidae Perameles nasuta Long-nosed Bandicoot Petauridae Petaurus australis Yellow Bellied Glider Petauridae Petaurus breviceps Sugar Glider Phalangeridae Trichosurus cunninghami Mountain Brushtail Possum Phalangeridae Trichosurus vulpecula Common Brushtail Possum Phascolarctidae Phascolarctos cinereus Koala Potoroidae Potorous sp. # ~ Long-nosed or Long-footed Potoroo Pseudocheiridae Petauroides volans Greater Glider Pseudocheiridae Pseudocheirus peregrinus -

How to Control Soil Insects with Beneficial Nematodes
How to Control Soil Insects with Beneficial Nematodes Ed Lewis Department of Entomology and Nematology University of California, Davis Using Microbials in IPM • Do not have to change everything about crop management • Many microbial insecticides fit into current production plans with minimal effort and change • They require specialized information about their use Insect pathogens can be effective • Naturally occur – Even in intensively managed systems • Have an impact on insect populations at natural levels Necessary information: Products • Shelf life • Storage conditions • Resting stage? • Viability in field • Host range • Time to kill • What does an infected insect look like? Recognized Species of Entomopathogenic Nematodes H. bacteriophora H. marelatus H. brevicaudis H. megidis H. hawaiiensis H. zealandica H. indica H. argentinensis S. kraussei S. karii S. arenarium S. kushidai S. bicornutum S. longicaudum S. carpocapsae S. monticolum S. caudatum S. neocurtillae S. ceratophorum S. oregonense S. cubanum S. puertoricense S. feltiae S. rarum S. glaseri S. riobrave S. intermedium S. ritteri S. affine S. scapterisci Infective Juveniles • Resistant to Environmental Extremes • Only Function is to Find A New Host • No Feeding • No Development • No Reproduction • Only Life Stage Outside the Host Infective Stage Juvenile Steinernema carpocapsae Symbiotic Bacteria Released Bacterial Chamber Mating for Steinernema spp. Two to three generations occur in a single host. About 6 days after the original infection, this is the appearance New Infective Juveniles in 10 Days Entomopathogenic Nematodes Can Control: • Weevils: Diaprepes root weevil, Diaprepes abbreviatus Blue green weevils, Pachnaeus spp. Otiorhynchus spp. Bill bugs • Fungus gnats: e.g., Sciaridae Entomopathogenic Nematodes Can Control: • Scarab larvae: e.g., Japanese beetle, Popillia japonica, Chafers, etc. -

210 Part 319—Foreign Quarantine Notices
§ 318.82–3 7 CFR Ch. III (1–1–03 Edition) The movement of plant pests, means of 319.8–20 Importations by the Department of conveyance, plants, plant products, and Agriculture. other products and articles from Guam 319.8–21 Release of cotton and covers after into or through any other State, Terri- 18 months’ storage. 319.8–22 Ports of entry or export. tory, or District is also regulated by 319.8–23 Treatment. part 330 of this chapter. 319.8–24 Collection and disposal of waste. 319.8–25 Costs and charges. § 318.82–3 Costs. 319.8–26 Material refused entry. All costs incident to the inspection, handling, cleaning, safeguarding, treat- Subpart—Sugarcane ing, or other disposal of products or ar- 319.15 Notice of quarantine. ticles under this subpart, except for the 319.15a Administrative instructions and in- services of an inspector during regu- terpretation relating to entry into Guam larly assigned hours of duty and at the of bagasse and related sugarcane prod- usual places of duty, shall be borne by ucts. the owner. Subpart—Citrus Canker and Other Citrus PART 319—FOREIGN QUARANTINE Diseases NOTICES 319.19 Notice of quarantine. Subpart—Foreign Cotton and Covers Subpart—Corn Diseases QUARANTINE QUARANTINE Sec. 319.24 Notice of quarantine. 319.8 Notice of quarantine. 319.24a Administrative instructions relating 319.8a Administrative instructions relating to entry of corn into Guam. to the entry of cotton and covers into Guam. REGULATIONS GOVERNING ENTRY OF INDIAN CORN OR MAIZE REGULATIONS; GENERAL 319.24–1 Applications for permits for impor- 319.8–1 Definitions. -

Four New Taxa of Acaulescent Syagrus (Arecaceae) from Brazil
Phytotaxa 188 (1): 001–013 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2014 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.188.1.1 Four new taxa of acaulescent Syagrus (Arecaceae) from Brazil LARRY R. NOBLICK1, HARRI LORENZI2 & VINICIUS C. SOUZA3 1 Montgomery Botanical Center, Miami, FL, U.S.A.; email: [email protected]; 2Jardim Plantarum, Nova Odessa, SP, Brazil, email: [email protected]; 3Departamento de Ciência Biológicas, ESALQ-USP, Piricicaba, SP, Brazil; email: [email protected] Abstract Three new species and a new subspecies of acaulescent Syagrus palms are described as new to science. These occur in the central western cerrado region of Brazil: Syagrus emasensis and S. menzeliana from southwestern Goiás, S. guimaraesensis from south central Mato Grosso and finally S. graminifolia subsp. cabraliensis from north central Minas Gerais. Keywords: Arecales, Arecoideae, Cocoseae, Palmae Introduction The genus Syagrus currently contains 56 species (Lorenzi et al. 2010, Noblick 2014, Soares et al. 2013). Of these 56 species, 26 are acaulescent (Noblick 2013). The word acaulescent translates as “without a trunk” and a trunk is defined as an above ground stem. While these palms appear to have no above ground stem, they do all have short, subterranean stems. Many of these acaulescent Syagrus are difficult to identify from herbarium material, but leaflet anatomy has been found to be useful in their identification (Glassman 1972, Noblick 2013). The discovery that the acaulescent Syagrus petraea (Mart.) Beccari (1916: 467) was a Bolivian endemic (Noblick et al. 2010), rather than the broadly distributed morphologically variable species that many botanists assumed that it was (Henderson et al. -

Plants That Have Everything but a Name
Daylilies at a Discount Big, Beautiful Plants That Have Everything but a Name We don't run a discount operation, but this time we summer color as a gardener is likely to get, and their have a bargain. It's our Daylily Mixture, which we call durability makes them ideal for naturalized plantings 'The Unique 50.' The mix contains 50 different Day where steep slopes or poor soil proscribe more demand lilies that offer a wide range of colors, forms, and ing ornamentals. In fact, Daylilies are the ideal way to blooming times. They're recent hybridizer crosses, turn wasteland into a wonderland, for less than a dollar bought in bulk from a pal who breeds them, and their per square foot. ancestry is as varied as one could ask. Colors range If this sounds like your kind of proposition, please or from the palest yellow to the deepest red, plus every der 'The Unique 50,' #83080, which includes 50 plants, nuance in between. Since each plant is unique, you will all blooming size, to be shipped in time for spring plant find some grand and glorious individuals that you can ing plus detailed cultural instructions. The price, $75, name after friends, an agreeable sort of compliment. is a fraction of the cost for 50 plants of named varieties, Yes, there may be a clunker or two (after all, these are which we hope will encourage an appropriate degree of the plants the breeder didn't keep), and they can be dis self-indulgence. Please add transportation charges of carded without regret. -

Causes and Consequences of Coati Sociality
chapter 28 Causes and consequences of coati sociality Ben T. Hirsch and Matthew E. Gompper Ring-tailed coatis (Nasua nasua) © B. Hirsch Introduction of Kaufmann’s work, and similar studies on pri- mates and other carnivores, have greatly enhanced Over fifty years ago John Kaufmann conducted a our understanding of how and why animals live in two-year study on the white-nosed coati (Nasua groups. Such issues frame the core of the modern narica) on Barro Colorado Island, Panama. The field of behavioural ecology. resulting monograph (Kaufmann 1962) is a solid Animals live in groups when the benefits (e.g. examination of the natural history of the species, a greater ability to survive threats from predators with an emphasis on understanding its social struc- and pathogens) are greater than the costs (e.g. in- ture. Although many such studies now exist, Kauf- creased competition for resources such as food or mann’s study bordered on revolutionary at the time mates) (Krause and Ruxton 2002). Overlaying such because this was one of the first studies to gather cost–benefit ratios are the genetic relatedness of in- detailed ethological data of wild vertebrates via dividuals and the willingness of animals to coop- habituation of free-living social animals. The idea erate in a manner that increases the benefits and of following animals from a distance of just a few decreases the costs of sociality. Among the mus- metres, and observing the nuances of their behav- teloid carnivores, studies of coatis have contrib- iour, was relatively novel at the time. The results uted more to our understanding of the causes and Hirsch, B. -

TAXON:Rhopalostylis Baueri SCORE:-2.0 RATING:Low Risk
TAXON: Rhopalostylis baueri SCORE: -2.0 RATING: Low Risk Taxon: Rhopalostylis baueri Family: Arecaceae Common Name(s): Norfolk Island palm Synonym(s): Areca baueri Hook. f. ex Lem. Eora(basionym) baueri (H. Wendl. & Drude) O. F. RhopalostylisCook cheesemanii Becc. ex Cheeseman Assessor: No Assessor Status: Assessor Approved End Date: WRA Score: -2.0 Designation: L Rating: Low Risk Keywords: Subtropical Palm, Unarmed, Shade-tolerant, Thicket-forming, Bird-dispersed Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 203 Broad climate suitability (environmental versatility) y=1, n=0 n Native or naturalized in regions with tropical or 204 y=1, n=0 y subtropical climates Does the species have a history of repeated introductions 205 y=-2, ?=-1, n=0 y outside its natural range? 301 Naturalized beyond native range y = 1*multiplier (see Appendix 2), n= question 205 n 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see Appendix 2) n 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see Appendix 2) n 304 Environmental weed n=0, y = 2*multiplier (see Appendix 2) n 305 Congeneric weed n=0, y = 1*multiplier