IR-9 Coordination Compounds (Draft March 2004)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inorganic Chemistry
Inorganic Chemistry Chemistry 120 Fall 2005 Prof. Seth Cohen Some Important Features of Metal Ions Electronic configuration • Oxidation State/Charge. •Size. • Coordination number. • Coordination geometry. • “Soft vs. Hard”. • Lability. • Electrochemistry. • Ligand environment. 1 Oxidation State/Charge • The oxidation state describes the charge on the metal center and number of valence electrons • Group - Oxidation Number = d electron count 3 4 5 6 7 8 9 10 11 12 Size • Size of the metal ions follows periodic trends. • Higher positive charge generally means a smaller ion. • Higher on the periodic table means a smaller ion. • Ions of the same charge decrease in radius going across a row, e.g. Ca2+>Mn2+>Zn2+. • Of course, ion size will effect coordination number. 2 Coordination Number • Coordination Number = the number of donor atoms bound to the metal center. • The coordination number may or may not be equal to the number of ligands bound to the metal. • Different metal ions, depending on oxidation state prefer different coordination numbers. • Ligand size and electronic structure can also effect coordination number. Common Coordination Numbers • Low coordination numbers (n =2,3) are fairly rare, in biological systems a few examples are Cu(I) metallochaperones and Hg(II) metalloregulatory proteins. • Four-coordinate is fairly common in complexes of Cu(I/II), Zn(II), Co(II), as well as in biologically less relevant metal ions such as Pd(II) and Pt(II). • Five-coordinate is also fairly common, particularly for Fe(II). • Six-coordinate is the most common and important coordination number for most transition metal ions. • Higher coordination numbers are found in some 2nd and 3rd row transition metals, larger alkali metals, and lanthanides and actinides. -

Introduction to Organic Chemistry 2018 More
Introduction to Organic Chemistry 25 Introduction to Organic Chemistry Handout 2 - Stereochemistry OH O O OH enantiomers Me OH HO Me A B NH2 NH2 diastereomers diastereomers diastereomers OH O O OH Me OH HO Me C D NH2 enantiomers NH2 http://burton.chem.ox.ac.uk/teaching.html ◼ Organic Chemistry J. Clayden, N. Greeves, S. Warren ◼ Stereochemistry at a Glance J. Eames & J. M. Peach ◼ The majority of organic chemistry text books have good chapters on the topics covered by these lectures ◼ Eliel Stereochemistry of Organic Compounds (advanced reference text) Introduction to Organic Chemistry 26 ◼ representations of formulae in organic chemistry ◼ skeletal representations are far less cluttered and as a result are much clearer than drawing all carbon and hydrogen atoms explicitly, they also give a much better representation of the likely bond angles and hence hybridisation states of the carbon atoms ◼ skeletal representations allow functional groups (sites of reactivity) to be clearly seen ◼ guidelines for drawing skeletal structures i) draw chains of atoms as zig-zags ii) do not draw C atoms unless there is good reason to draw them iii) do not draw C-H bonds unless there is good reason to draw then iv) do not draw Hs attached to carbon atoms unless there is good reason to draw them v) make drawings realistic Introduction to Organic Chemistry 27 ◼ representing structures in three dimensions ◼ a wedged bond indicates the bond is projecting out in front of the plane of the paper ◼ a dashed bond indicates the bond is projecting behind the plane -

Searching Coordination Compounds
CAS ONLINEB Available on STN Internationalm The Scientific & Technical Information Network SEARCHING COORDINATION COMPOUNDS December 1986 Chemical Abstracts Service A Division of the American Chemical Society 2540 Olentangy River Road P.O. Box 3012 Columbus, OH 43210 Copyright O 1986 American Chemical Society Quoting or copying of material from this publication for educational purposes is encouraged. providing acknowledgment is made of the source of such material. SEARCHING COORDINATION COMPOUNDS prepared by Adrienne W. Kozlowski Professor of Chemistry Central Connecticut State University while on sabbatical leave as a Visiting Educator, Chemical Abstracts Service Table of Contents Topic PKEFACE ............................s.~........................ 1 CHAPTER 1: INTRODUCTION TO SEARCHING IN CAS ONLINE ............... 1 What is Substructure Searching? ............................... 1 The Basic Commands .............................................. 2 CHAPTEK 2: INTKOOUCTION TO COORDINATION COPPOUNDS ................ 5 Definitions and Terminology ..................................... 5 Ligand Characteristics.......................................... 6 Metal Characteristics .................................... ... 8 CHAPTEK 3: STKUCTUKING AND REGISTKATION POLICIES FOR COORDINATION COMPOUNDS .............................................11 Policies for Structuring Coordination Compounds ................. Ligands .................................................... Ligand Structures........................................... Metal-Ligand -
![Ten-Coordinate Lanthanide [Ln(HL)(L)] Complexes (Ln = Dy, Ho, Er, Tb) with Pentadentate N3O2-Type Schiff-Base Ligands: Synthesis, Structure and Magnetism](https://docslib.b-cdn.net/cover/3551/ten-coordinate-lanthanide-ln-hl-l-complexes-ln-dy-ho-er-tb-with-pentadentate-n3o2-type-schi-base-ligands-synthesis-structure-and-magnetism-673551.webp)
Ten-Coordinate Lanthanide [Ln(HL)(L)] Complexes (Ln = Dy, Ho, Er, Tb) with Pentadentate N3O2-Type Schiff-Base Ligands: Synthesis, Structure and Magnetism
magnetochemistry Article Ten-Coordinate Lanthanide [Ln(HL)(L)] Complexes (Ln = Dy, Ho, Er, Tb) with Pentadentate N3O2-Type Schiff-Base Ligands: Synthesis, Structure and Magnetism Tamara A. Bazhenova 1, Ilya A. Yakushev 1,2,3 , Konstantin A. Lyssenko 4 , Olga V. Maximova 1,5,6, Vladimir S. Mironov 1,7,* , Yuriy V. Manakin 1 , Alexey B. Kornev 1, Alexander N. Vasiliev 5,8 and Eduard B. Yagubskii 1,* 1 Institute of Problems of Chemical Physics, 142432 Chernogolovka, Russia; [email protected] (T.A.B.); [email protected] (I.A.Y.); [email protected] (O.V.M.); [email protected] (Y.V.M.); [email protected] (A.B.K.) 2 Kurnakov Institute of General and Inorganic Chemistry, 119991 Moscow, Russia 3 National Research Center “Kurchatov Institute”, 123182 Moscow, Russia 4 Department of Chemistry, Lomonosov Moscow State University, 119991 Moscow, Russia; [email protected] 5 Faculty of Physics, Lomonosov Moscow State University, 119991 Moscow, Russia; [email protected] 6 National University “MISiS”, 119049 Moscow, Russia 7 Shubnikov Institute of Crystallography of Federal Scientific Research Centre “Crystallography and Photonics” RAS, 119333 Moscow, Russia 8 National Research South Ural State University, 454080 Chelyabinsk, Russia * Correspondence: [email protected] (V.S.M.); [email protected] (E.B.Y.) Received: 26 October 2020; Accepted: 9 November 2020; Published: 13 November 2020 Abstract: A series of five neutral mononuclear lanthanide complexes [Ln(HL)(L)] (Ln = Dy3+, 3+ 3+ 3+ H Ho Er and Tb ) with rigid pentadentate N3O2-type Schiff base ligands, H2L (1-Dy, OCH3 3-Ho, 4-Er and 6-Tb complexes) or H2L ,(2-Dy complex) has been synthesized by reaction H of two equivalents of 1,10-(pyridine-2,6-diyl)bis(ethan-1-yl-1-ylidene))dibenzohydrazine (H2L , [H2DAPBH]) or 1,10-(pyridine-2,6-diyl)bis(ethan-1-yl-1-ylidene))di-4-methoxybenzohydrazine OCH3 (H2L , [H2DAPMBH]) with common lanthanide salts. -

Bicapped Tetrahedral, Trigonal Prismatic, and Octahedral Alternatives in Main and Transition Group Six-Coordination
2484 Bicapped Tetrahedral, Trigonal Prismatic, and Octahedral Alternatives in Main and Transition Group Six-Coordination Roald Hoffmann,*laJames M. Howell,lb and Angelo R. RossilC Contribution from the Departments of Chemistry, Cornell University, Ithaca, New York 14853, Brooklyn College, City University of New York. Brooklyn, New York, 1121 0, and The University of Connecticut, Storrs, Connecticut 06268. Received June 30, 1975 Abstract: We examine theoretically the bicapped tetrahedron and trigonal prism as alternatives to the common octahedron for six-coordinate main and transition group compounds. The pronounced preference of six-coordinate sulfur for an octahe- dral geometry is traced by a molecular orbital analysis to a pair of nonbonding levels, whose higher energy in the nonoctahe- dral geometries is due to the molecular orbital equivalent of a ligand-ligand repulsion. For transition element complexes we draw a correlation diagram for the trigonal twist interrelating octahedral and trigonal prismatic extremes. A possible prefer- ence for the trigonal prism in systems with few d electrons is discussed, as well as a strategy for lowering the energy of the trigonal prism by symmetry-conditioned 7r bonding. The bicapped tetrahedron geometry for transition metal complexes can be stabilized by a substitution pattern ML4D2 where D is a good u donor and M is a d6 metal center. In main and transition group six-coordinate compounds logical, or group theoretical analysis of rearrangement the octahedral geometry predominates. There is, however, a modes or basic permutational sets. However, the solution of growing class of trigonal prismatic complexes and struc- the mathematical problem leaves the question of the physi- tures intermediate between the octahedron and trigonal cal mechanism of a rearrangement still unsolved. -

Metal Carbonyls
MODULE 1: METAL CARBONYLS Key words: Carbon monoxide; transition metal complexes; ligand substitution reactions; mononuclear carbonyls; dinuclear carbonyls; polynuclear carbonyls; catalytic activity; Monsanto process; Collman’s reagent; effective atomic number; 18-electron rule V. D. Bhatt / Selected topics in coordination chemistry / 2 MODULE 1: METAL CARBONYLS LECTURE #1 1. INTRODUCTION: Justus von Liebig attempted initial experiments on reaction of carbon monoxide with metals in 1834. However, it was demonstrated later that the compound he claimed to be potassium carbonyl was not a metal carbonyl at all. After the synthesis of [PtCl2(CO)2] and [PtCl2(CO)]2 reported by Schutzenberger (1868) followed by [Ni(CO)4] reported by Mond et al (1890), Hieber prepared numerous compounds containing metal and carbon monoxide. Compounds having at least one bond between carbon and metal are known as organometallic compounds. Metal carbonyls are the transition metal complexes of carbon monoxide containing metal-carbon bond. Lone pair of electrons are available on both carbon and oxygen atoms of carbon monoxide ligand. However, as the carbon atoms donate electrons to the metal, these complexes are named as carbonyls. A variety of such complexes such as mono nuclear, poly nuclear, homoleptic and mixed ligand are known. These compounds are widely studied due to industrial importance, catalytic properties and structural interest. V. D. Bhatt / Selected topics in coordination chemistry / 3 Carbon monoxide is one of the most important π- acceptor ligand. Because of its π- acidity, carbon monoxide can stabilize zero formal oxidation state of metals in carbonyl complexes. 2. SYNTHESIS OF METAL CARBONYLS Following are some of the general methods of preparation of metal carbonyls. -

Metal-Ligand Bonding and Inorganic Reaction Mechanisms Year 2
Metal-Ligand Bonding and Inorganic Reaction Mechanisms Year 2 RED Metal-ligand and metal-metal bonding of the transition metal elements Synopsis Lecture 1: Trends of the transition metal series. Ionic vs Covalent bonding. Nomenclature. Electron counting. Lecture 2: Thermodynamics of complex formation. Why complexes form. Recap of molecular orbital theory. 18-electron rule. Lecture 3: Ligand classes. -donor complexes. Octahedral ML6 molecular orbital energy diagram. Lecture 3: - acceptor ligands and synergic bonding. Binding of CO, CN , N2, O2 and NO. Lecture 4: Alkenes, M(H2) vs M(H)2, Mn(O2) complexes, PR3. Lecture 5: 2- - 2- 3- donor ligands, metal-ligand multiple bonds, O , R2N , RN , N . Lecture 6: ML6 molecular orbital energy diagrams incorporating acceptor and donor ligands. Electron counting revisited and link to spectrochemical series. Lecture 7: Kinetics of complex formation. Substitution mechanisms of inorganic complexes. Isomerisation. Lecture 8: Ligand effects on substitution rates (trans-effect, trans-influence). Metal and geometry effects on substitution rates. Lecture 9: Outer sphere electron transfer. Lecture 10: Inner Sphere electron transfer. Bridging ligands. 2 Learning Objectives: by the end of the course you should be able to i) Use common nomenclature in transition metal chemistry. ii) Count valence electrons and determine metal oxidation state in transition metal complexes. iii) Understand the physical basis of the 18-electron rule. iv) Appreciate the synergic nature of bonding in metal carbonyl complexes. v) Understand the relationship between CO, the 'classic' -acceptor and related ligands such as NO, CN, N2, and alkenes. 2 vi) Describe the nature of the interaction between -bound diatomic molecules (H2, O2) and their relationship to -acceptor ligands. -
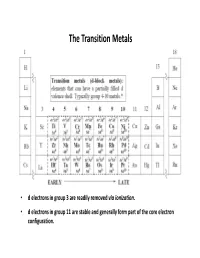
The Transition Metals
The Transition Metals • d electrons in group 3 are readily removed via ionization . • d electrons in group 11 are stable and generally form part of the core electron configuration. Transition Metal Valence Orbitals • nd orbitals • (n + 1)s and (n + 1)p orbitals • 2 2 2 dx -dy and dz (e g) lobes located on the axes • dxy, dxz, dyz lobes (t 2g) located between axes • orbitals oriented orthogonal wrt each other creating unique possibilities for ligand overlap. • Total of 9 valence orbitals available for bonding (2 x 9 = 18 valence electrons!) • For an σ bonding only Oh complex, 6 σ bonds are formed and the remaining d orbitals are non-bonding . • It's these non-bonding d orbitals that give TM complexes many of their unique properties • for free (gas phase) transition metals: (n+1)s is below (n)d in energy (recall: n = principal quantum #). • for complexed transition metals: the (n)d levels are below the (n+1)s and thus get filled first. (note that group # = d electron count) • for oxidized metals, subtract the oxidation state from the group #. Geometry of Transition Metals Coordination Geometry – arrangement of ligands around metal centre Valence Shell Electron Pair Repulsion (VSEPR) theory is generally not applicable to transition metals complexes (ligands still repel each other as in VSEPR theory) For example, a different geometry would be expected for metals of different d electron count 3+ 2 [V(OH 2)6] d 3+ 4 [Mn(OH 2)6] d all octahedral geometry ! 3+ 6 [Co(OH 2)6] d Coordination geometry is , in most cases , independent of ground state electronic configuration Steric: M-L bonds are arranged to have the maximum possible separation around the M. -

Synthesis and Structure of Two Octahedral Molybdenum Chloride Sulfide Clusters John Byrne Michel Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1979 Synthesis and structure of two octahedral molybdenum chloride sulfide clusters John Byrne Michel Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Inorganic Chemistry Commons Recommended Citation Michel, John Byrne, "Synthesis and structure of two octahedral molybdenum chloride sulfide clusters " (1979). Retrospective Theses and Dissertations. 7297. https://lib.dr.iastate.edu/rtd/7297 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This was produced from a copy of a document sent to us for microfilming. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help you understand markings or notations which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was posâble to obtain flie misâng page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure you of complete continuity. 2. When an image on the film is obliterated with a round black mark it is an indication that the film inspector noticed either blurred copy because of movement during exposure, or duplicate copy. -

Teaching with SCIGRESS
Teaching with SCIGRESS Exercises on Molecular Modeling in Chemistry Crispin Wong and James Currie, eds. Pacific University Teaching with SCIGRES Molecular Modeling in Chemistry Crispin Wong and James Currie Editors Pacific University Forest Grove, Oregon 2 Copyright statement © 2001 - 2012 Fujitsu Limited. All rights reserved. This document may not be used, sold, transferred, copied or reproduced, in any manner or form, or in any medium, to any person other than with the prior written consent of Fujitsu Limited. Published by Fujitsu Limited. All possible care has been taken in the preparation of this publication. Fujitsu Limited does not accept liability for any inaccuracies that may be found. Fujitsu Limited reserves the right to make changes without notice both to this publication and to the product it describes. 3 Table of Contents Exercises 7 Part 1: General Chemistry Experiment 1 Calculating the Geometry of Molecules and Ions 16 Experiment 2 Kinetics of Substitution Reactions 29 Experiment 3 Sunscreen and Ultraviolet Absorption 38 Part 2: Organic Chemistry Experiment 4 Evaluations of Conformations of Menthone and Isomenthone 46 Experiment 5 The Evelyn Effect 52 Part 3: Physical Chemistry Experiment 6 Investigating the Resonance Stabilization of Benzene 63 Experiment 7 Investigation of the Rotational Barrier in N-N-dimethylacetamide 68 Part 4: Inorganic Chemistry Experiment 8 Polarities of Small Molecules 75 Experiment 9 Structures of Molecules Which Exceed the Octet Rule. 77 Experiment 10 Comparison of Gas Phase Acidities of Binary -

Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013
International Union of Pure and Applied Chemistry Division VIII Chemical Nomenclature and Structure Representation Division Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013. Prepared for publication by Henri A. Favre and Warren H. Powell, Royal Society of Chemistry, ISBN 978-0-85404-182-4 Chapter P-9 SPECIFICATION OF CONFIGURATION AND CONFORMATION P-90 Introduction P-91 Stereoisomer graphical representation and naming P-92 The Cahn-Ingold-Prelog (CIP) priority system and the Sequence Rules P-93 Configuration specification P-94 Conformation and conformational stereodescriptors P-90 INTRODUCTION This Chapter is concerned only with the main principles for specification of configuration and conformation of organic compounds. The structure of an organic compound is systematically indicated by one or more affixes added to a name that does not itself prescribe stereochemical configuration or conformation; such affixes are generally called ‘stereodescriptors’. Thus, stereoisomers, such as enantiomers, have names that differ only in the stereodescriptors used. In contrast, ‘cis/trans’ isomers may have different names because of different stereodescriptors or names differing in the type of nomenclature. Also, certain retained names imply their own stereochemical description, for example, maleic acid, cholesterol, and other natural products described in Chapter P-10. In order to arrive at an unambiguous description of stereoisomers, Cahn, Ingold, and Prelog (refs. 34, 35) recommended an order of seniority for the ligands (atoms and groups) attached to carbon and other atoms, which is commonly called the ‘CIP priority system’. The priority is established by the application of ‘Sequence Rules’. These rules are discussed in P-92. Their application is then described for stereogenic units, mainly for the most usual compounds encountered in organic chemistry. -

New Ruthenium and Osmium Carbonyl Cluster Complexes with Main Group Bridging Ligands Having Unusual Structures and Bonding Yuwei Kan University of South Carolina
University of South Carolina Scholar Commons Theses and Dissertations 1-1-2013 New Ruthenium and Osmium Carbonyl Cluster Complexes With Main Group Bridging Ligands Having Unusual Structures and Bonding Yuwei Kan University of South Carolina Follow this and additional works at: https://scholarcommons.sc.edu/etd Part of the Chemistry Commons Recommended Citation Kan, Y.(2013). New Ruthenium and Osmium Carbonyl Cluster Complexes With Main Group Bridging Ligands Having Unusual Structures and Bonding. (Doctoral dissertation). Retrieved from https://scholarcommons.sc.edu/etd/2392 This Open Access Dissertation is brought to you by Scholar Commons. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. NEW RUTHENIUM AND OSMIUM CARBONYL CLUSTER COMPLEXES WITH MAIN GROUP BRIDGING LIGANDS HAVING UNUSUAL STRUCTURES AND BONDING by Yuwei Kan Bachelor of Engineering Jilin University, 2008 Submitted in Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy in Chemistry College of Arts & Sciences University of South Carolina 2013 Accepted by: Richard D. Adams, Major Professor Hans-Conrad Zur Loye, Committee Member Qian Wang, Committee Member Timir Datta, Committee Member Lacy Ford, Vice Provost and Dean of Graduate Studies © Copyright by Yuwei Kan, 2013 All Rights Reserved. ii ACKNOWLEDGEMENTS I would like to express my most sincere gratitude and appreciation to my advisor, Professor Richard Adams, for his guidance, patient and support throughout my research over the past five years. His dedication, passion and expertise in cluster chemistry made me always want to think and explore more. My sincere appreciation is extended to my committee members: Prof.