Battery-Like Supercapacitors from Vertically Aligned Carbon Nanofibers Coated Diamond: Design and Demonstrator
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review of Technologies and Materials Used in High-Voltage Film Capacitors
polymers Review Review of Technologies and Materials Used in High-Voltage Film Capacitors Olatoundji Georges Gnonhoue 1,*, Amanda Velazquez-Salazar 1 , Éric David 1 and Ioana Preda 2 1 Department of Mechanical Engineering, École de technologie supérieure, Montreal, QC H3C 1K3, Canada; [email protected] (A.V.-S.); [email protected] (É.D.) 2 Energy Institute—HEIA Fribourg, University of Applied Sciences of Western Switzerland, 3960 Sierre, Switzerland; [email protected] * Correspondence: [email protected] Abstract: High-voltage capacitors are key components for circuit breakers and monitoring and protection devices, and are important elements used to improve the efficiency and reliability of the grid. Different technologies are used in high-voltage capacitor manufacturing process, and at all stages of this process polymeric films must be used, along with an encapsulating material, which can be either liquid, solid or gaseous. These materials play major roles in the lifespan and reliability of components. In this paper, we present a review of the different technologies used to manufacture high-voltage capacitors, as well as the different materials used in fabricating high-voltage film capacitors, with a view to establishing a bibliographic database that will allow a comparison of the different technologies Keywords: high-voltage capacitors; resin; dielectric film Citation: Gnonhoue, O.G.; Velazquez-Salazar, A.; David, É.; Preda, I. Review of Technologies and 1. Introduction Materials Used in High-Voltage Film High-voltage films capacitors are important components for networks and various Capacitors. Polymers 2021, 13, 766. electrical devices. They are used to transport and distribute high-voltage electrical energy https://doi.org/10.3390/ either for voltage distribution, coupling or capacitive voltage dividers; in electrical sub- polym13050766 stations, circuit breakers, monitoring and protection devices; as well as to improve grid efficiency and reliability. -

New Polymer Dielectric for High Energy Density Film Capacitors
New Polymer Dielectric For High Energy Density Film Capacitors Paul Winsor, IV and Edward Lobo CDE 167 John Vertente Blv. New Bedford, MA 02745 Tel.: 508-994-9661 Fax: 508-995-3000 [email protected] [email protected] M. Zafar A. Munshi and Ayad Ibrahim Lithium Power Technolgies, Inc. 20955 Morris Avenue Manvel, TX 77578-3819 Tel.: 281-489-4889 Fax: 281-489-6644 [email protected] Abstract A new dielectric film has been developed that greatly increases the energy density capability of plastic film capacitors. This film has been developed over the past four years by a consortium consisting of Lithium Power Technologies, CDE Corp., Dupont Teijin Films (DTF), Case Western Reserve University, and Ohio Aerospace Institute in an Advanced Technology Program funded by the Department of Commerce. We are now entering the commercialization stage of this project. Plastic film capacitors have been the capacitor of choice for many power electronics, power conditioning, and pulse power applications such as motors, lighting and portable defibrillators because of their low dissipation factor (DF), excellent high-frequency response, high insulation resistance (IR), self-healing ability, and high-voltage capabilities. Existing capacitor technologies now present a barrier to achieving significant packaging (size and weight) reductions and struggle to meet new market-driven performance requirements. These markets not only include hybrid electric vehicles, wind power and portable defibrillators, but also the military and aerospace markets where the power source must be incredibly lightweight. Ultra thin gauge biaxially oriented films produced by DTF in thicknesses of 1.4 to 4 microns and thicker were constructed into metallized capacitors ranging from 1 to 140 microFarads. -

A Guide to Capacitors
The Handyman's Guide to CAPACITORS 32/<(67(5),/0&$3$&,7256 Polyester Films use layers of metal and polyester (Mylar â) dielectric to make a wide Capacitors comprise the largest variety of electronic components. There are range of capacitances in a many types of capacitors, great variations in their performance, many relatively small package at low methods of packaging and marking. and dozens of major manufacturers not voltages. These have become the to mention new types constantly being introduced with specific applications standard caps for DC applications and performances As result, capacitors open cause lots of problems for The "rolled" film layers cause homebrewers. Hopefully this article will take some of the mystery out of the high dissipation and capacitance myriad of capacitors available, plus present some of the classic "do's and vs. temperature problems and should be used carefully in high frequency or don'ts" high current applications 35,1&,3$/&$3$&,7257<3(6 There are many capacitor types, which usually refers to the material used 32/<3523(/(1(),/0&$3$&,7256 for the electrodes, dielectric, and the packaging or sealing method. Here are Polypropylene Films use layers of metal and polypropylene dielectric films some of the major capacitor types used by QRPers: virtually identical to Polyester Film caps. The polypropylene however, is a dielectric offering a higher breakdown voltage ',6.&(5$0,&&$3$&,7256 than polyester, and thus more suitable for high voltage Disk Ceramics consist of two metallic applications. such as switching power supplies. They also have plates separated by a ceramic low loss factors and good capacitance stability making them a dielectric whose area and spacing good choice for high frequency applications, including determines the capacitance. -

A Review of Degradation Behavior and Modeling of Capacitors: Preprint
A Review of Degradation Behavior and Modeling of Capacitors Preprint Anunay Gupta,1 Om Prakash Yadav,1 Douglas DeVoto,2 2 and Joshua Major 1 North Dakota State University 2 National Renewable Energy Laboratory Presented at the American Society of Mechanical Engineers (ASME) 2018 International Technical Conference and Exhibition on Packaging and Integration of Electronic and Photonic Microsystems (InterPACK 2018) San Francisco, California August 27–30, 2018 NREL is a national laboratory of the U.S. Department of Energy Conference Paper Office of Energy Efficiency & Renewable Energy NREL/CP-5400-71386 Operated by the Alliance for Sustainable Energy, LLC October 2018 This report is available at no cost from the National Renewable Energy Laboratory (NREL) at www.nrel.gov/publications. Contract No. DE-AC36-08GO28308 A Review of Degradation Behavior and Modeling of Capacitors Preprint Anunay Gupta,1 Om Prakash Yadav,1 Douglas DeVoto,2 2 and Joshua Major 1 North Dakota State University 2 National Renewable Energy Laboratory Suggested Citation Anunay Gupta, Om Prakash Yadav, Douglas DeVoto, and Joshua Major. 2018. A Review of Degradation Behavior and Modeling of Capacitors: Preprint. Golden, CO: National Renewable Energy Laboratory. NREL/CP-5400-71386. https://www.nrel.gov/docs/fy19osti/71386.pdf. NREL is a national laboratory of the U.S. Department of Energy Conference Paper Office of Energy Efficiency & Renewable Energy NREL/CP-5400-71386 Operated by the Alliance for Sustainable Energy, LLC October 2018 This report is available at no cost from the National Renewable Energy National Renewable Energy Laboratory Laboratory (NREL) at www.nrel.gov/publications. 15013 Denver West Parkway Golden, CO 80401 Contract No. -
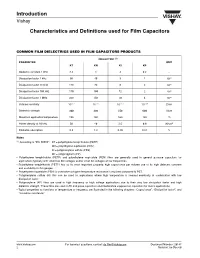
Introduction Characteristics and Definitions Used for Film Capacitors Vishay
Introduction Vishay Characteristics and Definitions used for Film Capacitors COMMON FILM DIELECTRICS USED IN FILM CAPACITORS PRODUCTS DIELECTRIC (1) PARAMETER UNIT KT KN KI KP Dielectric constant 1 kHz 3.3 3 3 2.2 - Dissipation factor 1 kHz 50 40 3 1 10-4 Dissipation factor 10 kHz 110 70 6 2 10-4 Dissipation factor 100 kHz 170 100 12 2 10-4 Dissipation factor 1 MHz 200 150 18 4 10-4 Volume resistivity 10+17 10+17 10+17 10+18 Ωcm Dielectric strength 400 300 250 600 V/µm Maximum application temperature 125 150 160 125 °C Power density at 10 kHz 50 40 2.5 0.6 W/cm3 Dielectric absorption 0.2 1.2 0.05 0.01 % Notes (1) According to “IEC 60062”: KT = polyethylene terephthalate (PETP) KN = polyethylene naphtalate (PEN) KI = polyphenylene sulfide (PPS) KP = polypropylene (PP) • Polyethylene terephthalate (PETP) and polyethylene naphtalate (PEN) films are generally used in general purpose capacitors for applications typically with small bias DC voltages and/or small AC voltages at low frequencies. • Polyethylene terephthalate (PETP) has as its most important property, high capacitance per volume due to its high dielectric constant and availability in thin gauges. • Polyethylene naphtalate (PEN) is used when a higher temperature resistance is required compared to PET. • Polyphenylene sulfide (KI) film can be used in applications where high temperature is needed eventually in combination with low dissipation factor. • Polypropylene (KP) films are used in high frequency or high voltage applications due to their very low dissipation factor and high dielectric strength. -

New Advances in Printed Circuits
New Advances in Printed Circuits United States Department of Commerce National Bureau of Standards Miscellaneous Publication 192 Printed Circuit Techniques . Printed circuits have emerged from purely laboratory experiments to become one of the most practical new ideas of mass production of electronic devices. Although many of the techniques were known and used long ago, printed circuits as we understand them today represent a comparatively recent accomplishment. Printed Circuit Techniques presents a comprehensive treatment of the subject. The scope of the book is indicated by the following chapters: Painting Die-Stamping Spraying Dusting Chemical Deposition Performance Vacuum Processes Applications . The book consists of ten chapters, totaling forty-three large, two-column pages. It is adequately illustrated with twenty-one halftones, eighteen line cuts, and five tables. Available from the Superintendent of Documents, U. S. Government Printing Office, Washington 25, D. C. Price: 25 cents a copy. UNITED STATES DEPARTMENT OF COMMERCE • Charles Sawyer, Secretary NATIONAL BUREAU OF STANDARDS • E. U. Condon, Director New Advances in Printed Circuits Proceedings of the First Technical Symposium on Printed Circuits held October 15, 1947, in Washington, D. C, under the Sponsorship of the Aeronautical Board and Technical Direction of the National Bureau of Standards Miscellaneous Publication 192 Issued November 22, 1948 For sale by the Superintendent of Documents, U. S. Government Printing Office, Washington 25, D. C. Price 40 cents PREFACE The wide interest shown in printed circuits by Government, scientific and industrial organizations throughout the country, since the first release of information by the National Bureau of Standards in February 1946, in- creased to the point where it was found desirable to call a general technical symposium for the purpose of bringing together all interested persons and affording a free and open presentation of the status, applications and limitations of the art. -

Low Temperature Characterization of Ceramic and Film Power Capacitors
https://ntrs.nasa.gov/search.jsp?R=19960045835 2020-06-16T04:15:35+00:00Z // . .. .'I"¸ _ _ /t NASA Technical Memorandum 107308 Low Temperature Characterization of Ceramic and Film Power Capacitors Ahmad Hammoud NYMA, Inc. Brook Park, Ohio and Eric Overton Lewis Research Center Cleveland, Ohio Prepared for the 1996 Conference on Electrical Insulation and Dielectric Phenomena sponsored by the IEEE Dielectrics and Electrical Insulation Society Millbrae, California, October 20-23, 1996 National Aeronautics and Space Administration Low Temperature Characterization of Ceramic and Film Power Capacitors Ahrnad Hammoud NYMA, Inc. NASA Lewis Research Group Cleveland, Ohio 44135 Eric Overton NASA Lewis Research Center Cleveland, Ohio 44135 Abstract and power systems deployed near Pluto will encounter temperatures as low as -229 °C [2]. The development Among the key requirements for advanced electronic of electrical components and systems capable of systems is the ability to withstand harsh environments extreme temperature operation represents, therefore, a while maintaining reliable and efficient operation. key element to meeting the technological challenges and Exposures to low temperature as well as high to fulfilling the requirements of advanced space power temperature constitute such stresses. Applications systems. In addition, the operation of advanced space where low temperatures are encountered include deep power electronics at low temperature will result in space missions, medical imaging equipment, and higher efficiency and lower cost (due to reduced active cryogenic instrumentation. Efforts were taken to design thermal management) [3]. and develop power capacitors capable of wide temperature operation. In this work, ceramic and film Commercially available power components are limited power capacitors were developed and characterized as in their temperature handling capability due to a function of temperature from 20 °C to -185 °C in limitations in the materials being used (semiconductor, terms of their dielectric properties. -

Plastic Film Capacitors Plastic Film Capacitors
PLASTIC FILM CAPACITORS PLASTIC FILM CAPACITORS Taped Capacitors for Automatic Insertion Systems (QYX) Metallized Polyester Film Capacitor QXK-(ZH) (Extended Standard Type) Type numbering system 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Highly reliable and superior performance in high frequency applications, self-healing and Q Y X 2 A 1 0 3 K T P 3 T A non-inductive construction, using a dielectric made of polyethylene terephthalate film covered with vacuum-evaporated metal. Packaging (A : standard) Finished by inner dipping with liquid epoxy resin and outer coating with flame-retardant T : Ammo - pack epoxy resin, those double coating provides excellent humidity resistance. 3 : Formed lead type Designed to be compact and to cover larger capacitance range having advantage of Representing taping style ( ) 5 : Straight lead type tolerating to A.C.voltage and large current flow. Designed 1mm max. of epoxy on lead wire for best performance at soldering process on Taping specifications P.C. board assemblies. Δh Δh P2 P Compliant to the RoHS directive (2011/65/EU,(EU)2015/863). Applications Filtering, DC-blocking, coupling and so on of general communications equipment and use in AC circuits for motor starting, charging / discharging, lighting, noise suppression and etc. MAX. Contact us for details for use in AC circuits. P1 F 1 H 0 2 However, do not use this product for across-the-line applications. H w t 1 H L 0 w w w P0 d D0 Specifications Type numbering system (Example : 630V 0.01µF) Item Performance Characteristics 1 2 3 4 5 6 7 8 9 10 11 12 13 14 -

Metallized Film Capacitor Lifetime Evaluation and Failure Mode Analysis
Metallized Film Capacitor Lifetime Evaluation and Failure Mode Analysis R. Gallay Garmanage, Farvagny-le-Petit, Switzerland Abstract One of the main concerns for power electronic engineers regarding capacitors is to predict their remaining lifetime in order to anticipate costly failures or system unavailability. This may be achieved using a Weibull statistical law combined with acceleration factors for the temperature, the voltage, and the humidity. This paper discusses the different capacitor failure modes and their effects and consequences. Keywords Metallized film capacitor; failure mode; lifetime. 1 Capacitor technologies The following different power capacitor technologies are used in inverters: – Electrolytic capacitors characterized by very big capacitance per volume unit, but with low rated voltages and very important power losses due to the ionic conductivity. In particular, the bigger the capacitance density, the lower the rated voltage. – Film foil capacitors made of dielectric films between two plain aluminium foils. These capacitors can sustain very high currents. – Metallized film capacitors, which are made with dielectric films with a metallic coating on the surface. With this technology the electric-field stress may be much bigger than with film capacitors thanks to the metallization self-healing capability. Today the dielectric films that are used are mainly polypropylene (PP) or polyethylene terephthalate (PET). Formerly, paper (PA) was used in film foil technology—either pure paper or mixed with polypropylene (DM). In special applications, where high temperatures are required, polyethylene naphthalene (PEN) up to 125°C or polyphenylene sulfide (PPS) up to 150°C are used. PET presents the following advantages over PP: a dielectric constant 50% bigger (ε = 3.3 versus 2.2), which means 50% more capacitance in the same volume, a better mechanical resistance (which means a higher endurance to self-healing), and the possibility of manipulating thinner films, consequently leading to a smaller capacitance and a higher exploitation temperature (+10°C). -

Solen Teflon ™ Fep Film & Tin Foil Capacitors
SOLEN TEFLON ™ FEP FILM & TIN FOIL CAPACITORS • GENERAL INFORMATION Type : Tin Foil / Teflon™ FEP Film Capacitor. Dielectric : Biaxially Oriented Teflon™ FEP Film. Construction : Round Tubular "Square Aspect Ratio" Type, Axial Leads. Coating : Green Plastic Tape Wrapped, Grey Epoxy Resin Sealed. Electrodes : Pure Tin Foil. Winding : Bifilar Extended Foil Design. Contact : Non-Inductive, Radially Knurled Extended Foil. Connectors : Tinned Plated Oxygen Free Pure Copper. • TECHNICAL DATA Capacitance Range/Tolerance : .033 ... .82 uF, E 24 series, +-5 %, typ. 2% (see specifications for details) Dielectric Constant : 2.0 er, non-polar dielectric. Dielectric Absorbtion Factor : Less Than .05 % @ 20°C. Equivalent Series Resistance : Extremely Low (see specifications for details) Dissipation Factor : Extremely Low (see specifications for details) Self Inductance : Less Than 12 nH with 6 mm leads. Insulation Resistance : More than 100,000 MegOhm @ 20°C. Temperature Range : -40°C to +130°C. Test Voltage : 1.5 x Vr for 5 sec. Rated Voltage : 1000 VDC/400 VAC. Dielectric Thickness : 5.5 micron, 10 micron. Metal Layers Thickness : 5 micron Leads Diameter : 1.2, 1.8 mm pure copper.. (see specifications for details) • FEATURE Special Tubular "Square Aspect Ratio" Type Construction. Very High Conductivity Tin Foil. Tin/Silver Soldered Lead Termination. Very High Current Capacity. High Frequency and Temperature Stability. Excellent Long Term Electrical and Mechanical Reliability. No Short Term and / or Long Term Signal Aberation. Unrivaled Handling of Fast High Current Pulse. • ELECTRICAL PERFORMANCE Very Low Dielectric Absortion Factor. Very Low Equivalent Series Resistance. Very Low Self Inductance. Very High Self Resonant Frequency Ultra Linear Impedance Characteristics. Very Low Dissipation Factor. Very High Insulation Resistance. -

Full Range of Film Capacitors for Audio & Video: Audiocap™ Multi Cap Exotica™
Full Range of Film Capacitors for Audio & Video: AudioCap™ Multi Cap™ Exotica™ ■ Film 14 Foil aluminum. tin. copper. polypropylene, polystyrene, Teflon Metalized Polypropylene .001 • 100 Microfarads 100 • .2400 Volts Reliable Capacitors • MultiCap RTX - Ultimate Film & Foil Recommended for high-current applications and wherever the finest performance is required. This poly Incorporated in 1962, Rel Cap has become a leading and innovative styrene cap exhibits the lowest DA and the highest stability in frequency producer of plastic film dielectric capacitors.Custom designed caps are and temperature characteristics. Its tin foil is compatible with the other now a part of the company's standard capabilities. Rel Cap has long materials used in the MultiCap , preventing oxidation and electrolysis and produced capacitors for the telecommunication, computer, industrial, providing an extraordinarily stable long-term performance, and consumerOEM markets and includes a division specializingin her metically sealed capacitors for military, aerospace, instrumentation & • MultiCap PPFXS - Excellent Film & Foil Recommended for high high-voltage applications, and high temperature usage in oil drilling, current, high-temperature applications. Polypropylene & tin, with power supplymanufacture, and for nuclear environments. similar characteristics to RTX, but with extended temperature range. Rel now introduces a division devoted to audio and video manufactur • MultiCap PPFX - Excellent Film & Foil Recommended for high ers, modiiiers, and distributors. In theseproducts, the company's abilities current, high-temperature applications. Polypropylene & aluminum. in the fields above are combined and concentrated into speciallines that Lowest ESR available. Lighter in weight, lower in cost than the RTX will meet the most exacting audio and video 'f)ecifications. or PPFXS. Highly accurate, stable performance. -

MLCC C0G Guide for Resonance Circuits Vol
Solution Guide (1/4) MLCC(Multilayer Ceramic Capacitors)・C0G Guide for Resonance Circuits Vol.1 Features of High voltage MLCCs with C0G Characteristics and Replacement Solutions A wide variety of capacitors, each with their own special characteristics, are used in electronic devices. Generally speaking, the capacitance and withstand voltage (rated voltage) of capacitors are in a trade-off relationship which is difficult to balance. In MLCC of the same size, when increasing the withstand voltage, the capacitance tends to decrease. Film capacitors possess a good balance of high withstand voltage and capacitance. Since they also possess outstanding frequency characteristics and temperature characteristics, they are widely used in automotive electronics, industrial equipment, home appliances, etc. However, in recent years, there have been remarkable increases in withstand voltage and capacitance in MLCCs (multilayer ceramic chip capacitors) for temperature compensation (type 1). In particular, even in fields where film capacitors have traditionally been used, resonance circuits for example, replacement with MLCC is now possible. TDK has developed high voltage MLCCs with C0G characteristics. Through C0G characteristics, these MLCCs achieve withstand voltage of 1000V at the broadest capacitance range (1nF to 33nF) in the industry. In this guide, we explain the numerous benefits of replacement while comparing the features of high voltage C0G MLCCs with those of film capacitors. Characteristics of main capacitors MLCCs are divided into two major categories according to the type of ceramic materials used for their dielectric, namely type 1 (temperature compensating) and type 2 (high dielectric constant). Type 2 MLCCs have a large capacitance. However, they also have a disadvantage in terms of a large capacitance change caused by temperature.