Tunable Polytetrafluoroethylene Electret Films with Extraordinary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introduction to Electrets: Principles, Equations, Experimental Techniques
Introduction to electrets: Principles, equations, experimental techniques Gerhard M. Sessler Darmstadt University of Technology Institute for Telecommunications Merckstrasse 25, 64283 Darmstadt, Germany [email protected] Darmstadt University of Technology • Institute for Telecommunications Overview Principles Charges Materials Electret classes Equations Fields Forces Currents Charge transport Experimental techniques Charging Surface potential Thermally-stimulated discharge Dielectric measurements Charge distribution (surface) Charge distribution (volume) Darmstadt University of Technology • Institute for Telecommunications Electret charges Darmstadt University of Technology • Institute for Telecommunications Energy diagram and density of states for a polymer Darmstadt University of Technology • Institute for Telecommunications Electret materials Polymers Anorganic materials Fluoropolymers (PTFE, FEP) Silicon oxide (SiO 2) Polyethylene (HDPE, LDPE, XLPE) Silicon nitride (Si 3N4) Polypropylene (PP) Aluminum oxide (Al 2O3) Polyethylene terephtalate (PET) Glas (SiO 2 + Na, S, Se, B, ...) Polyimid (PI) Photorefractive materials Polymethylmethacrylate (PMMA) • Polyvinylidenefluoride (PVDF) • Ethylene vinyl acetate (EVA) • • • Cellular and porous polymers Cellular PP Porous PTFE Darmstadt University of Technology • Institute for Telecommunications Charged or polarized dielectrics Category Materials Charge or polarization Properties Applications Density Geometry [mC/m2 ] Real-charge External electric FEP, SiO electrets 2 0.1 - 1 field -

Review of Technologies and Materials Used in High-Voltage Film Capacitors
polymers Review Review of Technologies and Materials Used in High-Voltage Film Capacitors Olatoundji Georges Gnonhoue 1,*, Amanda Velazquez-Salazar 1 , Éric David 1 and Ioana Preda 2 1 Department of Mechanical Engineering, École de technologie supérieure, Montreal, QC H3C 1K3, Canada; [email protected] (A.V.-S.); [email protected] (É.D.) 2 Energy Institute—HEIA Fribourg, University of Applied Sciences of Western Switzerland, 3960 Sierre, Switzerland; [email protected] * Correspondence: [email protected] Abstract: High-voltage capacitors are key components for circuit breakers and monitoring and protection devices, and are important elements used to improve the efficiency and reliability of the grid. Different technologies are used in high-voltage capacitor manufacturing process, and at all stages of this process polymeric films must be used, along with an encapsulating material, which can be either liquid, solid or gaseous. These materials play major roles in the lifespan and reliability of components. In this paper, we present a review of the different technologies used to manufacture high-voltage capacitors, as well as the different materials used in fabricating high-voltage film capacitors, with a view to establishing a bibliographic database that will allow a comparison of the different technologies Keywords: high-voltage capacitors; resin; dielectric film Citation: Gnonhoue, O.G.; Velazquez-Salazar, A.; David, É.; Preda, I. Review of Technologies and 1. Introduction Materials Used in High-Voltage Film High-voltage films capacitors are important components for networks and various Capacitors. Polymers 2021, 13, 766. electrical devices. They are used to transport and distribute high-voltage electrical energy https://doi.org/10.3390/ either for voltage distribution, coupling or capacitive voltage dividers; in electrical sub- polym13050766 stations, circuit breakers, monitoring and protection devices; as well as to improve grid efficiency and reliability. -

Note PERFORMANCE of ELECTRET IONIZATION CHAMBERS IN
Note PERFORMANCE OF ELECTRET IONIZATION CHAMBERS IN MAGNETIC FIELD P. Kotrappa,* L. R. Stieff,* T. F. Mengers,† and R. D. Shull† The change in charge is measured using a portable charge Abstract—Electret ionization chambers are widely used for reader and is the measure of the integrated ionization measuring radon and radiation. The radiation measured in- cludes alpha, beta, and gamma radiation. These detectors do over the sampling period. These chambers are widely not have any electronics and as such can be introduced into used for measuring radon in air (Kotrappa et al. 1990) magnetic field regions. It is of interest to study the effect of and environmental gamma radiation (Fjeld et al. 1994; magnetic fields on the performance of these detectors. Relative Hobbs et al. 1996). These chambers are also used for responses are measured with and without magnetic fields present. Quantitative responses are measured as the magnetic measuring alpha and beta contamination levels (Kasper field is varied from 8 kA/m to 716 kA/m (100 to 9,000 gauss). 1999; Kotrappa et al. 1995). One feature of these No significant effect is observed for measuring alpha radiation detectors, which makes them unique, is that there are no and gamma radiation. However, a significant systematic effect electronic components or power supply associated with is observed while measuring beta radiation from a 90Sr-Y source. Depending upon the field orientation, the relative the detectors. Because of this property, these detectors response increased from 1.0 to 2.7 (vertical position) and can be used in areas with magnetic fields present without decreased from 1.0 to 0.60 (horizontal position). -

How to Make an Electret the Device That Permanently Maintains an Electric Charge by C
How to Make an Electret the Device That Permanently Maintains an Electric Charge by C. L. Strong Scientific America, November, 1960 Danger Level 4: (POSSIBLY LETHAL!!) Alternative Science Resources World Clock Synthesis Home --------------------- THE HISTORY OF SCIENCE IS A TREASURE house for the amateur experimenter. For example, many devices invented by early workers in electricity and magnetism attract little attention today because they have no practical application, yet these devices remain fascinating in themselves. Consider the so-called electret. This device is a small cake of specially prepared wax that has the property of permanently maintaining an electric field; it is the electrical analogue of a permanent magnet. No one knows in precise detail how an electret works, nor does it presently have a significant task to perform. George O. Smith, an electronics specialist of Rumson, N.J., points out, however, that this is no obstacle to the enjoyment of the electret by the amateur. Moreover, the amateur with access to a source of high-voltage current can make an electret at virtually no cost. "For more than 2,000 years," writes Smith, "it was suspected that the magnetic attraction of the lodestone and the electrostatic attraction of the electrophorus were different manifestations of the same phenomenon. This suspicion persisted from the time of Thales of Miletus (600 B.C.) to that of William Gilbert (A.D. 1600). After the publication of Gilbert's treatise De Magnete, the suspicion graduated into a theory that was supported by many experiments conducted to show that for every magnetic effect there was an electric analogue, and vice versa. -

Electret Nanogenerators for Self-Powered, Flexible Electronic Pianos
sustainability Article Electret Nanogenerators for Self-Powered, Flexible Electronic Pianos Yongjun Xiao 1, Chao Guo 2, Qingdong Zeng 1, Zenggang Xiong 1, Yunwang Ge 2, Wenqing Chen 2, Jun Wan 3,4,* and Bo Wang 2,* 1 School of Physics and Electronic-Information Engineering, Hubei Engineering University, Xiaogan 432000, China; [email protected] (Y.X.); [email protected] (Q.Z.); [email protected] (Z.X.) 2 School of Electrical Engineering and Automation, Luoyang Institute of Science and Technology, Luoyang 471023, China; [email protected] (C.G.); [email protected] (Y.G.); [email protected] (W.C.) 3 State Key Laboratory for Hubei New Textile Materials and Advanced Processing Technology, Wuhan Textile University, Wuhan 430200, China 4 Hubei Key Laboratory of Biomass Fiber and Ecological Dyeing and Finishing, School of Chemistry and Chemical Engineering, Wuhan Textile University, Wuhan 430200, China * Correspondence: [email protected] (J.W.); [email protected] (B.W.) Abstract: Traditional electronic pianos mostly adopt a gantry type and a large number of rigid keys, and most keyboard sensors of the electronic piano require additional power supply during playing, which poses certain challenges for portable electronic products. Here, we demonstrated a fluorinated ethylene propylene (FEP)-based electret nanogenerator (ENG), and the output electrical performances of the ENG under different external pressures and frequencies were systematically characterized. At a fixed frequency of 4 Hz and force of 4 N with a matched load resistance of 200 MW, an output 2 power density of 20.6 mW/cm could be achieved. Though the implementation of a signal processing circuit, ENG-based, self-powered pressure sensors have been demonstrated for self-powered, flexible Citation: Xiao, Y.; Guo, C.; Zeng, Q.; electronic pianos. -

Charge Storage in Electret Polymers: Mechanisms, Characterization and Applications
Charge Storage in Electret Polymers: Mechanisms, Characterization and Applications Habilitationsschrift zur Erlangung des akademischen Grades doctor rerum naturalium habilitatus (Dr. rer. nat. habil.) der Mathematisch-Naturwissenschaftlichen Fakult¨at der Universit¨at Potsdam vorgelegt von Dr. Axel Mellinger geb. am 25. August 1967 in M¨unchen Potsdam, 06. Dezember 2004 iii Abstract Electrets are materials capable of storing oriented dipoles or an electric surplus charge for long periods of time. The term “electret” was coined by Oliver Heaviside in analogy to the well-known word “magnet”. Initially regarded as a mere scientific curiosity, electrets became increasingly imporant for applications during the second half of the 20th century. The most famous example is the electret condenser microphone, developed in 1962 by Sessler and West. Today, these devices are produced in annual quantities of more than 1 billion, and have become indispensable in modern communications technology. Even though space-charge electrets are widely used in transducer applications, relatively little was known about the microscopic mechanisms of charge storage. It was generally accepted that the surplus charges are stored in some form of physical or chemical traps. However, trap depths of less than 2 eV, obtained via thermally stimulated discharge experiments, conflicted with the observed lifetimes (extrapolations of experimental data yielded more than 100 000 years). Using a combination of photostimulated discharge spectroscopy and simultaneous depth-profiling of the space-charge density, the present work shows for the first time that at least part of the space charge in, e. g., polytetrafluoroethylene, polypropylene and polyethylene terephthalate is stored in traps with depths of up to 6 eV, indicating major local structural changes. -

Piezoelectricity in Cellular Electret Films
IEEE Transactions on Dielectrics and Electrical Insulation Vol. 7 No. 4, August 2000 537 Piezoelectricity in Cellular Electret Films J. Hillenbrand and G. M. Sessler Institute for Communications Technology Darmstadt University of Technology Darmstadt, Germany ABSTRACT Permanently charged films with a cellular or porous structure represent a new family of poly- mer electrets. These materials show piezoelectric properties with high piezoelectric constants. The electromechanical response equations of such films are derived for their operation as sen- sors and as actuators. Experimental results are also presented for cellular polypropylene (PP). In particular, measurements of the direct and inverse piezoelectric constants in the frequency range 0 to 10 kHz and of the variation of these constants across the surface of the films are dis- cussed. These measurements, performed by direct application of stress or by the use of a pro- filometer, an accelerometer and an interferometer yield a frequency-independent piezoelectric $33 constant of 5 220 pC1N. Assuming reasonable charge distributions and charge densities, the calculated piezoelectric constants are in good agreement with the measured values. The theoretical model shows the reciprocity of the piezoelectric constants. 1 INTRODUCTION The charged material, electroded on top and bottom, consists of plane parallel solid layers and air layers of thicknesses SI, and sa,, respec- HERE has been considerable recent interest in the permanent tively, with n = 1,2,.. iV and m, = I, 2, . N - I, where N is charging of films with a cellular or porous structure [l-151. It T the total number of solid layers. It is further assumed that the two solid was shown that such films of polypropylene (PP), polytetrafluoroethy- surfaces confining the m-th air layer carry a total planar charge density lene, and silicon dioxide, when charged with corona or other methods, of om and -oTn,respectively, and that no volume charges exist. -

New Polymer Dielectric for High Energy Density Film Capacitors
New Polymer Dielectric For High Energy Density Film Capacitors Paul Winsor, IV and Edward Lobo CDE 167 John Vertente Blv. New Bedford, MA 02745 Tel.: 508-994-9661 Fax: 508-995-3000 [email protected] [email protected] M. Zafar A. Munshi and Ayad Ibrahim Lithium Power Technolgies, Inc. 20955 Morris Avenue Manvel, TX 77578-3819 Tel.: 281-489-4889 Fax: 281-489-6644 [email protected] Abstract A new dielectric film has been developed that greatly increases the energy density capability of plastic film capacitors. This film has been developed over the past four years by a consortium consisting of Lithium Power Technologies, CDE Corp., Dupont Teijin Films (DTF), Case Western Reserve University, and Ohio Aerospace Institute in an Advanced Technology Program funded by the Department of Commerce. We are now entering the commercialization stage of this project. Plastic film capacitors have been the capacitor of choice for many power electronics, power conditioning, and pulse power applications such as motors, lighting and portable defibrillators because of their low dissipation factor (DF), excellent high-frequency response, high insulation resistance (IR), self-healing ability, and high-voltage capabilities. Existing capacitor technologies now present a barrier to achieving significant packaging (size and weight) reductions and struggle to meet new market-driven performance requirements. These markets not only include hybrid electric vehicles, wind power and portable defibrillators, but also the military and aerospace markets where the power source must be incredibly lightweight. Ultra thin gauge biaxially oriented films produced by DTF in thicknesses of 1.4 to 4 microns and thicker were constructed into metallized capacitors ranging from 1 to 140 microFarads. -

A Guide to Capacitors
The Handyman's Guide to CAPACITORS 32/<(67(5),/0&$3$&,7256 Polyester Films use layers of metal and polyester (Mylar â) dielectric to make a wide Capacitors comprise the largest variety of electronic components. There are range of capacitances in a many types of capacitors, great variations in their performance, many relatively small package at low methods of packaging and marking. and dozens of major manufacturers not voltages. These have become the to mention new types constantly being introduced with specific applications standard caps for DC applications and performances As result, capacitors open cause lots of problems for The "rolled" film layers cause homebrewers. Hopefully this article will take some of the mystery out of the high dissipation and capacitance myriad of capacitors available, plus present some of the classic "do's and vs. temperature problems and should be used carefully in high frequency or don'ts" high current applications 35,1&,3$/&$3$&,7257<3(6 There are many capacitor types, which usually refers to the material used 32/<3523(/(1(),/0&$3$&,7256 for the electrodes, dielectric, and the packaging or sealing method. Here are Polypropylene Films use layers of metal and polypropylene dielectric films some of the major capacitor types used by QRPers: virtually identical to Polyester Film caps. The polypropylene however, is a dielectric offering a higher breakdown voltage ',6.&(5$0,&&$3$&,7256 than polyester, and thus more suitable for high voltage Disk Ceramics consist of two metallic applications. such as switching power supplies. They also have plates separated by a ceramic low loss factors and good capacitance stability making them a dielectric whose area and spacing good choice for high frequency applications, including determines the capacitance. -

A Review of Degradation Behavior and Modeling of Capacitors: Preprint
A Review of Degradation Behavior and Modeling of Capacitors Preprint Anunay Gupta,1 Om Prakash Yadav,1 Douglas DeVoto,2 2 and Joshua Major 1 North Dakota State University 2 National Renewable Energy Laboratory Presented at the American Society of Mechanical Engineers (ASME) 2018 International Technical Conference and Exhibition on Packaging and Integration of Electronic and Photonic Microsystems (InterPACK 2018) San Francisco, California August 27–30, 2018 NREL is a national laboratory of the U.S. Department of Energy Conference Paper Office of Energy Efficiency & Renewable Energy NREL/CP-5400-71386 Operated by the Alliance for Sustainable Energy, LLC October 2018 This report is available at no cost from the National Renewable Energy Laboratory (NREL) at www.nrel.gov/publications. Contract No. DE-AC36-08GO28308 A Review of Degradation Behavior and Modeling of Capacitors Preprint Anunay Gupta,1 Om Prakash Yadav,1 Douglas DeVoto,2 2 and Joshua Major 1 North Dakota State University 2 National Renewable Energy Laboratory Suggested Citation Anunay Gupta, Om Prakash Yadav, Douglas DeVoto, and Joshua Major. 2018. A Review of Degradation Behavior and Modeling of Capacitors: Preprint. Golden, CO: National Renewable Energy Laboratory. NREL/CP-5400-71386. https://www.nrel.gov/docs/fy19osti/71386.pdf. NREL is a national laboratory of the U.S. Department of Energy Conference Paper Office of Energy Efficiency & Renewable Energy NREL/CP-5400-71386 Operated by the Alliance for Sustainable Energy, LLC October 2018 This report is available at no cost from the National Renewable Energy National Renewable Energy Laboratory Laboratory (NREL) at www.nrel.gov/publications. 15013 Denver West Parkway Golden, CO 80401 Contract No. -
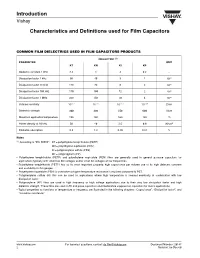
Introduction Characteristics and Definitions Used for Film Capacitors Vishay
Introduction Vishay Characteristics and Definitions used for Film Capacitors COMMON FILM DIELECTRICS USED IN FILM CAPACITORS PRODUCTS DIELECTRIC (1) PARAMETER UNIT KT KN KI KP Dielectric constant 1 kHz 3.3 3 3 2.2 - Dissipation factor 1 kHz 50 40 3 1 10-4 Dissipation factor 10 kHz 110 70 6 2 10-4 Dissipation factor 100 kHz 170 100 12 2 10-4 Dissipation factor 1 MHz 200 150 18 4 10-4 Volume resistivity 10+17 10+17 10+17 10+18 Ωcm Dielectric strength 400 300 250 600 V/µm Maximum application temperature 125 150 160 125 °C Power density at 10 kHz 50 40 2.5 0.6 W/cm3 Dielectric absorption 0.2 1.2 0.05 0.01 % Notes (1) According to “IEC 60062”: KT = polyethylene terephthalate (PETP) KN = polyethylene naphtalate (PEN) KI = polyphenylene sulfide (PPS) KP = polypropylene (PP) • Polyethylene terephthalate (PETP) and polyethylene naphtalate (PEN) films are generally used in general purpose capacitors for applications typically with small bias DC voltages and/or small AC voltages at low frequencies. • Polyethylene terephthalate (PETP) has as its most important property, high capacitance per volume due to its high dielectric constant and availability in thin gauges. • Polyethylene naphtalate (PEN) is used when a higher temperature resistance is required compared to PET. • Polyphenylene sulfide (KI) film can be used in applications where high temperature is needed eventually in combination with low dissipation factor. • Polypropylene (KP) films are used in high frequency or high voltage applications due to their very low dissipation factor and high dielectric strength. -

New Advances in Printed Circuits
New Advances in Printed Circuits United States Department of Commerce National Bureau of Standards Miscellaneous Publication 192 Printed Circuit Techniques . Printed circuits have emerged from purely laboratory experiments to become one of the most practical new ideas of mass production of electronic devices. Although many of the techniques were known and used long ago, printed circuits as we understand them today represent a comparatively recent accomplishment. Printed Circuit Techniques presents a comprehensive treatment of the subject. The scope of the book is indicated by the following chapters: Painting Die-Stamping Spraying Dusting Chemical Deposition Performance Vacuum Processes Applications . The book consists of ten chapters, totaling forty-three large, two-column pages. It is adequately illustrated with twenty-one halftones, eighteen line cuts, and five tables. Available from the Superintendent of Documents, U. S. Government Printing Office, Washington 25, D. C. Price: 25 cents a copy. UNITED STATES DEPARTMENT OF COMMERCE • Charles Sawyer, Secretary NATIONAL BUREAU OF STANDARDS • E. U. Condon, Director New Advances in Printed Circuits Proceedings of the First Technical Symposium on Printed Circuits held October 15, 1947, in Washington, D. C, under the Sponsorship of the Aeronautical Board and Technical Direction of the National Bureau of Standards Miscellaneous Publication 192 Issued November 22, 1948 For sale by the Superintendent of Documents, U. S. Government Printing Office, Washington 25, D. C. Price 40 cents PREFACE The wide interest shown in printed circuits by Government, scientific and industrial organizations throughout the country, since the first release of information by the National Bureau of Standards in February 1946, in- creased to the point where it was found desirable to call a general technical symposium for the purpose of bringing together all interested persons and affording a free and open presentation of the status, applications and limitations of the art.