Rapid Detection of Orthopoxvirus by Semi-Nested PCR Directly from Clinical Specimens: a Useful Alternative for Routine Laboratories
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VACCINIA VIRUS O1L VIRULENCE GENE and PROTEIN LOCALIZATION by Shayna Mooney a Senior Honors Project Presented to the Honors Co
VACCINIA VIRUS O1L VIRULENCE GENE AND PROTEIN LOCALIZATION by Shayna Mooney A Senior Honors Project Presented to the Honors College East Carolina University In Partial Fulfillment of the Requirements for Graduation with Honors by Shayna Mooney Greenville, NC May 2015 Approved by: Dr. Rachel Roper Department of Microbiology and Immunology, Brody School of Medicine Mooney 2 Abstract Smallpox killed an estimated 500 million people in the twentieth century alone. Although this fatal disease was eradicated from the world over thirty years ago, its potential use as a bioterrorism agent remains a concern. In addition, monkeypox continues to cause human outbreaks in Africa, and in the US in 2003. Vaccinia virus, the live virus vaccine for smallpox and monkeypox, is dangerous for immunocompromised individuals, and a safer vaccine is needed. The Roper lab studies how poxviruses cause disease in mammals and which genes contribute to virulence. The vaccinia virus O1L gene is highly conserved in poxviruses, and we have shown that it is required for full virulence in mice. When the O1L gene is removed from the wild type virus, the virus becomes attenuated, and immune responses are improved. Very little is known about this protein including its molecular weight, location within the cell and its function. We raised anti O1L peptide antibodies in rabbits and are using these to investigate the localization of the O1L protein using immunofluorescence techniques. In accordance with preliminary data from western blot analysis, we hypothesized that the O1L protein is located in the nucleus of the cell. Through immunofluorescence, the O1L protein was detected in the nucleus and cytoplasm of the cell. -

Risk Groups: Viruses (C) 1988, American Biological Safety Association
Rev.: 1.0 Risk Groups: Viruses (c) 1988, American Biological Safety Association BL RG RG RG RG RG LCDC-96 Belgium-97 ID Name Viral group Comments BMBL-93 CDC NIH rDNA-97 EU-96 Australia-95 HP AP (Canada) Annex VIII Flaviviridae/ Flavivirus (Grp 2 Absettarov, TBE 4 4 4 implied 3 3 4 + B Arbovirus) Acute haemorrhagic taxonomy 2, Enterovirus 3 conjunctivitis virus Picornaviridae 2 + different 70 (AHC) Adenovirus 4 Adenoviridae 2 2 (incl animal) 2 2 + (human,all types) 5 Aino X-Arboviruses 6 Akabane X-Arboviruses 7 Alastrim Poxviridae Restricted 4 4, Foot-and- 8 Aphthovirus Picornaviridae 2 mouth disease + viruses 9 Araguari X-Arboviruses (feces of children 10 Astroviridae Astroviridae 2 2 + + and lambs) Avian leukosis virus 11 Viral vector/Animal retrovirus 1 3 (wild strain) + (ALV) 3, (Rous 12 Avian sarcoma virus Viral vector/Animal retrovirus 1 sarcoma virus, + RSV wild strain) 13 Baculovirus Viral vector/Animal virus 1 + Togaviridae/ Alphavirus (Grp 14 Barmah Forest 2 A Arbovirus) 15 Batama X-Arboviruses 16 Batken X-Arboviruses Togaviridae/ Alphavirus (Grp 17 Bebaru virus 2 2 2 2 + A Arbovirus) 18 Bhanja X-Arboviruses 19 Bimbo X-Arboviruses Blood-borne hepatitis 20 viruses not yet Unclassified viruses 2 implied 2 implied 3 (**)D 3 + identified 21 Bluetongue X-Arboviruses 22 Bobaya X-Arboviruses 23 Bobia X-Arboviruses Bovine 24 immunodeficiency Viral vector/Animal retrovirus 3 (wild strain) + virus (BIV) 3, Bovine Bovine leukemia 25 Viral vector/Animal retrovirus 1 lymphosarcoma + virus (BLV) virus wild strain Bovine papilloma Papovavirus/ -

Here, There, and Everywhere: the Wide Host Range and Geographic Distribution of Zoonotic Orthopoxviruses
viruses Review Here, There, and Everywhere: The Wide Host Range and Geographic Distribution of Zoonotic Orthopoxviruses Natalia Ingrid Oliveira Silva, Jaqueline Silva de Oliveira, Erna Geessien Kroon , Giliane de Souza Trindade and Betânia Paiva Drumond * Laboratório de Vírus, Departamento de Microbiologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais: Belo Horizonte, Minas Gerais 31270-901, Brazil; [email protected] (N.I.O.S.); [email protected] (J.S.d.O.); [email protected] (E.G.K.); [email protected] (G.d.S.T.) * Correspondence: [email protected] Abstract: The global emergence of zoonotic viruses, including poxviruses, poses one of the greatest threats to human and animal health. Forty years after the eradication of smallpox, emerging zoonotic orthopoxviruses, such as monkeypox, cowpox, and vaccinia viruses continue to infect humans as well as wild and domestic animals. Currently, the geographical distribution of poxviruses in a broad range of hosts worldwide raises concerns regarding the possibility of outbreaks or viral dissemination to new geographical regions. Here, we review the global host ranges and current epidemiological understanding of zoonotic orthopoxviruses while focusing on orthopoxviruses with epidemic potential, including monkeypox, cowpox, and vaccinia viruses. Keywords: Orthopoxvirus; Poxviridae; zoonosis; Monkeypox virus; Cowpox virus; Vaccinia virus; host range; wild and domestic animals; emergent viruses; outbreak Citation: Silva, N.I.O.; de Oliveira, J.S.; Kroon, E.G.; Trindade, G.d.S.; Drumond, B.P. Here, There, and Everywhere: The Wide Host Range 1. Poxvirus and Emerging Diseases and Geographic Distribution of Zoonotic diseases, defined as diseases or infections that are naturally transmissible Zoonotic Orthopoxviruses. Viruses from vertebrate animals to humans, represent a significant threat to global health [1]. -

Discovery of Antivirals Against Smallpox
Discovery of antivirals against smallpox Stephen C. Harrisona,b, Bruce Albertsc, Ellie Ehrenfeldd, Lynn Enquiste, Harvey Finebergf, Steven L. McKnightg, Bernard Mossh, Michael O’Donnelli, Hidde Ploeghj, Sandra L. Schmidk, K. Peter Walterl, and Julie Theriotm aHarvard Medical School, Howard Hughes Medical Institute, Seeley Mudd Building, Room 130, 250 Longwood Avenue, Boston, MA 02115; cNational Academy of Sciences, 2101 Constitution Avenue, NW, Washington, DC 20418; dLaboratory of Infectious Disease, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Building 50, Room 6120, 50 South Drive, Bethesda, MD 20892; ePrinceton University, 314 Schultz Laboratory, Washington Road, Princeton, NJ 08544; fInstitute of Medicine, 2101 Constitution Avenue, NW, Washington, DC 20418; gDepartment of Biochemistry, University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX 75390; hLaboratory of Viral Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Building 4, Room 229, 4 Center Drive, Bethesda, MD 20892; iLaboratory of DNA Replication, The Rockefeller University, Howard Hughes Medical Institute, 1230 York Avenue, New York, NY 10021; jDepartment of Pathology, Harvard Medical School, NRB, 77 Avenue Louis Pasteur, Boston, MA 02115; kDepartment of Cell Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037; lDepartment of Biochemistry and Biophysics, University of California School of Medicine, Howard Hughes Medical Institute, Box 0448, HSE 1001, San Francisco, CA 94143; and mDepartment of Biochemistry, Stanford University School of Medicine, Stanford, CA 94305 Contributed by Stephen C. Harrison, May 21, 2004 mallpox, a devastating infectious Whatever the likelihood of covertly dopoxviruses has a restricted and spe- disease dreaded throughout much held variola virus stocks, an intentional cific host array (Table 2). -

The Current and Future Landscape of Smallpox Vaccines
Lane JM. The current and future landscape of smallpox vaccines. Global Biosecurity, 2019; 1(1). REVIEWS The current and future landscape of smallpox vaccines J Michael Lane1 1 Emeritus Professor of Preventive Medicine, Emory University, Atlanta, Georgia, USA. Abstract Smallpox is a potential weapon for bioterrorism. There is a need for better smallpox vaccines. The first generation vaccines such as Dryvax were made using crude methods that would not allow licensure today. Second generation vaccines, grown in modern tissue cultures but employing seed virus from first generation vaccines, have been developed. One, ACAM2000, has been licensed and added to the US National Stockpile. These second generation vaccines can produce the same complications as first generation vaccines. Myopericarditis has been well documented as caused by ACAM2000. This has created advocacy for third and fourth generation smallpox vaccines. Third generation vaccines are viruses that have been attenuated by serial passage in non-human cells, or by careful laboratory deletions of selected genes. Two of these, Modified Vaccinia Ankara, and LC16m8, derived from Lister strain vaccinia, have been tested in human trials. These seem to be ready to apply for licensure if there proves to be a market. Fourth generation vaccines, created in the laboratory as subunits of full-strength vaccinia, or fully engineered non-replicating molecules that express various epitopes of vaccinia and/or smallpox, have also been developed. Proving the efficacy of these vaccines may be difficult because smallpox no longer exists and there is no animal model that accurately reflects the human disease. These fourth generation vaccines include large DNA viruses into which immunogens from others agents such as HIV and malaria can be inserted. -

Review Article When Good Vaccines Go Wild: Feral Orthopoxvirus in Developing Countries and Beyond
Review Article When good vaccines go wild: Feral Orthopoxvirus in developing countries and beyond Nissin Moussatché,1,2 Clarissa R. Damaso,2 and Grant McFadden1 1Dept. Molecular Genetics and Microbiology, University of Florida, Gainesville, FL 32610-0266, United States of America. 2Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, 21941-902, Brazil. Abstract The presence of zoonotic poxviruses in nature represents a potential human health risk that has to be re-evaluated by health authorities not only in developing countries, but also in many developed countries. For example, buffalopox virus infection remains to be a threat to humans and cattle in India, and monkeypox virus infection persists in several inhabited places in Africa and, more recently, in the USA. There are also a great number of zoonotic transmissions of cowpox virus from cats to humans in Europe. For almost a decade in Brazil, vaccinia-like viruses have been isolated from human and cattle infections. This review examines the ability of potentially pathogenic orthopoxviruses, including feral versions of vaccinia virus vaccine, to persist in nature and re-emerge for reasons we do not yet understand. Key Words: Poxviridae, Buffalopox, Brazilian vaccinia-like virus, cowpox, monkeypox, vaccinia, variola, smallpox vaccine, zoonotic virus, orthopoxvirus in humans. J Infect Developing Countries 2008; 2(3):156-173. Received 12 March 2008 - Accepted 30 April 2008 Copyright © 2008 Moussatché et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. -

Chapter 29. Human Monkeypox and Other Poxvirus Infections of Man
CHAPTER 29 HUMAN MONKEYPOX AND OTHER POXVIRUS INFECTIONS OF MAN Contents Page Introduction 1287 Monkeypox in captive primates 1288 The properties of monkeypox virus 1290 Pathogenicity for laboratory animals 1290 Comparison of DNA maps of strains of monkeypox virus 1290 Genetic studies 1291 Species diagnosis 1292 Serological diagnosis of past monkeypox infection 1292 Human monkeypox 1292 Discovery of human infections 1292 Organization of laboratory research 1293 Organization of field research 1295 Incidence and distribution 1295 Clinical features 1297 Epidemiology 1303 Ecological studies 1308 Monkeypox : the overall picture 1311 Other Orthopoxvirus infections of man 1311 Vaccinia 1311 Cowpox 1312 Camelpox 1316 Parapoxvirus infections 1316 Molluscum contagiosum 1317 Tanapox virus infections 1318 General comment 1319 INTRODUCTION mild symptoms and usually only a localized skin lesion, the diseases in question presented Human monkeypox was first recognized in a potential diagnostic problem during the 1970 ; it is a severe systemic disease with a global eradication of smallpox, since virus generalized pustular rash, clinically indistin- particles found in lesions by electron micro- guishable from smallpox. In addition to va- scopic examination could be confused with riola and monkeypox viruses, 7 other species those of variola virus. Because of its import- of poxvirus, of 4 genera, can cause lesions in ance, monkeypox is the main subject of this man (Table 29 .1). Although infection with chapter, but a brief description is given of each of -

Human Monkeypox Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention
Human Monkeypox Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention a,b,c, d Eskild Petersen, MD, DMSc, DTMH *, Anu Kantele, MD, PhD , e f,g Marion Koopmans, DVM, PhD , Danny Asogun, MBBS, FWACP , h Adesola Yinka-Ogunleye, BDS, MPH , h Chikwe Ihekweazu, MBBS, MPH, FFPH , i Alimuddin Zumla, FRCP, PhD, FRCPath KEYWORDS Monkeypox Smallpox West Africa Epidemic KEY POINTS Human monkeypox is a zoonosis caused by the monkeypox virus (MPXV), a double- stranded DNA virus of the family Poxviridae. The frequency and geographic distribution of human monkeypox cases across West and Central Africa have increased in recent years. Continued All authors contributed equally. A. Zumla, D. Asogun, and C. Ihekweazu are members of the PANDORA-ID-NET Consortium. PANDORA-ID-NET (EDCTP Reg/Grant RIA2016E-1609) is funded by the European and Devel- oping Countries Clinical Trials Partnership (EDCTP2) programme, which is supported under Ho- rizon 2020, the European Union’s Framework Programme for Research and Innovation. A. Zumla is in receipt of a National Institutes of Health Research senior investigator award. Conflicts of Interest: All authors have an interest in global public health and emerging and re- emerging infections. All authors have no other conflict of interest to declare. a Institute of Clinical Medicine, University of Aarhus, Palle Juul-Jensens Boulevard 82, Aarhus N DK-8200, Denmark; b The Royal Hospital, Muscat, Oman; c European Society for Clinical Microbiology and Infectious Diseases, Task Force for Emerging Infections, -
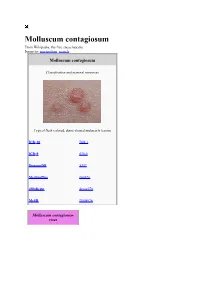
Molluscum Contagiosum from Wikipedia, the Free Encyclopedia Jump To: Navigation, Search
Molluscum contagiosum From Wikipedia, the free encyclopedia Jump to: navigation, search Molluscum contagiosum Classification and external resources Typical flesh-colored, dome-shaped and pearly lesions ICD-10 B 08.1 ICD-9 078.0 DiseasesDB 8337 MedlinePlus 000826 eMedicine derm/270 MeSH D008976 Molluscum contagiousm virus EM of Molluscum contagiosum virus Virus classification Group: Group I (dsDNA) Family: Poxviridae Genus: Molluscipoxvirus Molluscum Species: contagiosum virus Molluscum contagiosum (MC) is a viral infection of the skin or occasionally of the mucous membranes. It is caused by a DNA poxvirus called the molluscum contagiosum virus (MCV). MCV has no animal reservoir, infecting only humans. There are four types of MCV, MCV-1 to -4; MCV-1 is the most prevalent and MCV-2 is seen usually in adults and often sexually transmitted. This common viral disease has a higher incidence in children, sexually active adults, and those who are immunodeficient,[1] and the infection is most common in children aged one to ten years old.[2] MC can affect any area of the skin but is most common on the trunk of the body, arms, and legs. It is spread through direct contact or shared items such as clothing or towels. The virus commonly spreads through skin-to-skin contact. This includes sexual contact or touching or scratching the bumps and then touching the skin. Handling objects that have the virus on them (fomites), such as a towel, can also result in infection. The virus can spread from one part of the body to another or to other people. The virus can be spread among children at day care or at school. -

Case Report of Laboratory- Acquired Vaccinia Virus Infection in India Cas D'infection En Laboratoire Par Le Virus De La Vaccin
Case report of laboratory- Cas d’infection en laboratoire acquired vaccinia virus par le virus de la vaccine infection in India en Inde Renuka Nawadkar,a Ameet Dravid,b Umesh Paprunia,c Renuka Nawadkar,a Ameet Dravid,b Umesh Paprunia,c Rosamund Lewis,d Kazunobu Kojima,e Priya Abrahamf Rosamund Lewis,d Kazunobu Kojima,e Priya Abrahamf et and Arvind Sahua Arvind Sahua Introduction Introduction Various strains of vaccinia virus (VACV), Les vaccins qui ont été utilisés dans le monde a prototype of the genus Orthopoxvirus, entier pour éradiquer la variole contenaient were used around the world in vaccines to diverses souches du virus de la vaccine eradicate smallpox.1 The World Health (VACV), un prototype du genre Orthopoxvi- Assembly recommended termination of rus.1 Après l’éradication de la maladie en 1980, vaccination against smallpox after eradi- l’Assemblée mondiale de la Santé a recom- cation of the disease in 1980.2 mandé de mettre un terme à la vaccination antivariolique.2 During vaccination campaigns with Lors des campagnes de vaccination utilisant des vaccinia-based smallpox vaccines, the vaccins antivarioliques à base de virus de la virus was shown to infect domestic vaccine, il a été constaté que le virus infectait les animals, particularly dairy cattle.3 Over animaux domestiques, en particulier le bétail time, VACV evolved into buffalopox, laitier.3 Au fil du temps, le VACV a évolué pour which caused infections in both cattle aboutir au buffalopox (virus de la variole du and people.4 Brazil and India have buffle), lequel peut infecter aussi bien le bétail reported repeated outbreaks in dairy que l’homme.4 Le Brésil et l’Inde ont signalé des cattle that led to zoonotic infections in flambées répétées chez le bétail laitier, qui ont humans. -

Virus-Encoded Complement Regulators: Current Status
viruses Review Virus-Encoded Complement Regulators: Current Status Anwesha Sinha 1, Anup Kumar Singh 1, Trupti Satish Kadni 1, Jayati Mullick 2 and Arvind Sahu 1,* 1 Complement Biology Laboratory, National Centre for Cell Science, S. P. Pune University Campus, Ganeskhind, Pune 411007, India; [email protected] (A.S.); [email protected] (A.K.S.); [email protected] (T.S.K.) 2 Polio Virology Group, Microbial Containment Complex, ICMR-National Institute of Virology, Pune 411021, India; [email protected] * Correspondence: [email protected]; Tel.: +91-20-2570-8083; Fax: +91-20-2569-2259 Abstract: Viruses require a host for replication and survival and hence are subjected to host immuno- logical pressures. The complement system, a crucial first response of the host immune system, is effective in targeting viruses and virus-infected cells, and boosting the antiviral innate and acquired immune responses. Thus, the system imposes a strong selection pressure on viruses. Consequently, viruses have evolved multiple countermeasures against host complement. A major mechanism employed by viruses to subvert the complement system is encoding proteins that target complement. Since viruses have limited genome size, most of these proteins are multifunctional in nature. In this review, we provide up to date information on the structure and complement regulatory functions of various viral proteins. Keywords: viral immune evasion; complement; innate immunity; pathogenesis; Poxvirus; Herpesvirus; RCA; CD59; viral RCA Citation: Sinha, A.; Singh, A.K.; 1. Introduction Kadni, T.S.; Mullick, J.; Sahu, A. Viruses face constant challenges from the host due to host resistance, and the environ- Virus-Encoded Complement ment because of variations in temperature and humidity. -

Recent Worldwide Research on Animal Pox Viruses
OpenȱSourceȱCenter Analysis Recent Worldwide Research on Animal Pox Viruses January 2008 This peer-reviewed scientific assessment was prepared for the Open Source Center by the MITRE Corporation. Principal author Dr Alfred D. Steinberg, MD is a consulting physician-scientist for MITRE, where he provides advice to government agencies on molecular genetics, immunology, microbiology, bioterrorism, and remote sensing of human infectious diseases and animal/plant pathogens. A member of the National Intelligence Council's 2015 Threat Panel 2000-2002 and current member of the Department of Homeland Security's oversight committee on biological warning and informatics systems, Dr Steinberg was Chief of the Cellular Immunology Section at NIH from 1981 to 1992. Dr Steinberg joined the MITRE Corporation in 1992 and he is currently Senior Consulting Physician-Scientist. He has published over 480 papers, many in the areas of immunology, molecular genetics, and microbiology, and has served on the editorial boards for the Journal of Immunology, Journal of Immunopharmacology, African Journal of Clinical Immunology, Clinical and Experimental Rheumatology, Journal of Autoimmunity, and Acta Pathologica, Microbiologica, et Immunologica Scandinavica. Dr Steinberg earned his MD from Harvard Medical School. 1 Executive Summary Although smallpox disease has been eliminated worldwide, concerns remain regarding variola virus (the cause of smallpox) and related poxviruses. It is widely feared that samples of variola virus may have been retained in several countries after a WHO directive to allow storage of such samples only in Russia and the United States. Even if the US and Russian stocks of variola virus were now destroyed, there would still be concern over possible hidden collections.