Evidence from Organismal Physiology and Gene Expression
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Animal Species with Ranks October 2017
Washington Natural Heritage Program List of Animal Species with Ranks October 2017 The following list of animals known from Washington is complete for resident and transient vertebrates and several groups of invertebrates, including odonates, branchipods, tiger beetles, butterflies, gastropods, freshwater bivalves and bumble bees. Some species from other groups are included, especially where there are conservation concerns. Among these are the Palouse giant earthworm, a few moths and some of our mayflies and grasshoppers. Currently 857 vertebrate and 1,100 invertebrate taxa are included. Conservation status, in the form of range-wide, national and state ranks are assigned to each taxon. Information on species range and distribution, number of individuals, population trends and threats is collected into a ranking form, analyzed, and used to assign ranks. Ranks are updated periodically, as new information is collected. We welcome new information for any species on our list. Common Name Scientific Name Class Global Rank State Rank State Status Federal Status Northwestern Salamander Ambystoma gracile Amphibia G5 S5 Long-toed Salamander Ambystoma macrodactylum Amphibia G5 S5 Tiger Salamander Ambystoma tigrinum Amphibia G5 S3 Ensatina Ensatina eschscholtzii Amphibia G5 S5 Dunn's Salamander Plethodon dunni Amphibia G4 S3 C Larch Mountain Salamander Plethodon larselli Amphibia G3 S3 S Van Dyke's Salamander Plethodon vandykei Amphibia G3 S3 C Western Red-backed Salamander Plethodon vehiculum Amphibia G5 S5 Rough-skinned Newt Taricha granulosa -

Robert Wood Dissertation Final(1)
PSYCHO-SPIRITUAL TRANSFORMATION EXPERIENCED BY PARTICIPANTS OF MODERN WILDERNESS RITES OF PASSAGE QUESTS: AN INTUITIVE INQUIRY by Robert Wood A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Psychology Institute of Transpersonal Psychology Palo Alto, California March 7, 2010 I certify that I have read and approved the content and presentation of this dissertation: ________________________________________________ ____________ Nancy Rowe, Ph.D., Committee Chairperson Date ________________________________________________ ____________ Charles Fisher, Ph.D., Committee Member Date ________________________________________________ ____________ John Davis, Ph.D., Committee Member Date UMI Number: 3397618 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. UMI 3397618 Copyright 2010 by ProQuest LLC. All rights reserved. This edition of the work is protected against unauthorized copying under Title 17, United States Code. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, MI 48106-1346 Copyright © Robert Wood 2010 All Rights Reserved ii Abstract Psycho-Spiritual Transformation Experienced by Participants of Modern Wilderness Rites of Passage Quests: An Intuitive Inquiry by Robert Wood This study investigates and reports the quest experiences of modern wilderness rites of passage questers that prompted psycho-spiritual transformation, the nature of those psycho-spiritual transformations, and the context of questers’ lives when called to quest. Intuitive Inquiry provided the method for the research that relied on the researcher’s intuitive impressions of interviews with 12 questers who believed they experienced psycho-spiritual transformation as the result of a quest. -

(Trichoptera: Limnephilidae) in Western North America By
AN ABSTRACT OF THE THESIS OF Robert W. Wisseman for the degree of Master ofScience in Entomology presented on August 6, 1987 Title: Biology and Distribution of the Dicosmoecinae (Trichoptera: Limnsphilidae) in Western North America Redacted for privacy Abstract approved: N. H. Anderson Literature and museum records have been reviewed to provide a summary on the distribution, habitat associations and biology of six western North American Dicosmoecinae genera and the single eastern North American genus, Ironoquia. Results of this survey are presented and discussed for Allocosmoecus,Amphicosmoecus and Ecclisomvia. Field studies were conducted in western Oregon on the life-histories of four species, Dicosmoecusatripes, D. failvipes, Onocosmoecus unicolor andEcclisocosmoecus scvlla. Although there are similarities between generain the general habitat requirements, the differences or variability is such that we cannot generalize to a "typical" dicosmoecine life-history strategy. A common thread for the subfamily is the association with cool, montane streams. However, within this stream category habitat associations range from semi-aquatic, through first-order specialists, to river inhabitants. In feeding habits most species are omnivorous, but they range from being primarilydetritivorous to algal grazers. The seasonal occurrence of the various life stages and voltinism patterns are also variable. Larvae show inter- and intraspecificsegregation in the utilization of food resources and microhabitatsin streams. Larval life-history patterns appear to be closely linked to seasonal regimes in stream discharge. A functional role for the various types of case architecture seen between and within species is examined. Manipulation of case architecture appears to enable efficient utilization of a changing seasonal pattern of microhabitats and food resources. -

Microsoft Outlook
Joey Steil From: Leslie Jordan <[email protected]> Sent: Tuesday, September 25, 2018 1:13 PM To: Angela Ruberto Subject: Potential Environmental Beneficial Users of Surface Water in Your GSA Attachments: Paso Basin - County of San Luis Obispo Groundwater Sustainabilit_detail.xls; Field_Descriptions.xlsx; Freshwater_Species_Data_Sources.xls; FW_Paper_PLOSONE.pdf; FW_Paper_PLOSONE_S1.pdf; FW_Paper_PLOSONE_S2.pdf; FW_Paper_PLOSONE_S3.pdf; FW_Paper_PLOSONE_S4.pdf CALIFORNIA WATER | GROUNDWATER To: GSAs We write to provide a starting point for addressing environmental beneficial users of surface water, as required under the Sustainable Groundwater Management Act (SGMA). SGMA seeks to achieve sustainability, which is defined as the absence of several undesirable results, including “depletions of interconnected surface water that have significant and unreasonable adverse impacts on beneficial users of surface water” (Water Code §10721). The Nature Conservancy (TNC) is a science-based, nonprofit organization with a mission to conserve the lands and waters on which all life depends. Like humans, plants and animals often rely on groundwater for survival, which is why TNC helped develop, and is now helping to implement, SGMA. Earlier this year, we launched the Groundwater Resource Hub, which is an online resource intended to help make it easier and cheaper to address environmental requirements under SGMA. As a first step in addressing when depletions might have an adverse impact, The Nature Conservancy recommends identifying the beneficial users of surface water, which include environmental users. This is a critical step, as it is impossible to define “significant and unreasonable adverse impacts” without knowing what is being impacted. To make this easy, we are providing this letter and the accompanying documents as the best available science on the freshwater species within the boundary of your groundwater sustainability agency (GSA). -

Naturalist Pocket Reference
Table of Contents Naturalist Phone Numbers 1 Park info 5 Pocket GRTE Statistics 6 Reference Timeline 8 Name Origins 10 Mountains 12 Things to Do 19 Hiking Trails 20 Historic Areas 23 Wildlife Viewing 24 Visitor Centers 27 Driving Times 28 Natural History 31 Wildlife Statistics 32 Geology 36 Grand Teton Trees & Flowers 41 National Park Bears 45 revised 12/12 AM Weather, Wind Scale, Metric 46 Phone Numbers Other Emergency Avalanche Forecast 733-2664 Bridger-Teton Nat. Forest 739-5500 Dispatch 739-3301 Caribou-Targhee NF (208) 524-7500 Out of Park 911 Grand Targhee Resort 353-2300 Jackson Chamber of Comm. 733-3316 Recorded Information Jackson Fish Hatchery 733-2510 JH Airport 733-7682 Weather 739-3611 JH Mountain Resort 733-2292 Park Road Conditions 739-3682 Information Line 733-2291 Wyoming Roads 1-888-996-7623 National Elk Refuge 733-9212 511 Post Office – Jackson 733-3650 Park Road Construction 739-3614 Post Office – Moose 733-3336 Backcountry 739-3602 Post Office – Moran 543-2527 Campgrounds 739-3603 Snow King Resort 733-5200 Climbing 739-3604 St. John’s Hospital 733-3636 Elk Reduction 739-3681 Teton Co. Sheriff 733-2331 Information Packets 739-3600 Teton Science Schools 733-4765 Wyoming Game and Fish 733-2321 YELL Visitor Info. (307) 344-7381 Wyoming Highway Patrol 733-3869 YELL Roads (307) 344-2117 WYDOT Road Report 1-888-442-9090 YELL Fill Times (307) 344-2114 YELL Visitor Services 344-2107 YELL South Gate 543-2559 1 3 2 Concessions AMK Ranch 543-2463 Campgrounds - Colter Bay, Gros Ventre, Jenny Lake 543-2811 Campgrounds - Lizard Creek, Signal Mtn. -

Jacksonhole Skier 2009
JacksonHole skier 2009 WINTER VISITOR’S GUIDE FREE . Inviting you to our 35th Anniversary Shearling Sale 20 W. Broadway Downtown Jackson Across from the Wort Hotel Est. 1973 www.jollyjumbuckleathers.com 307.733.6562 Experience the Old West in a New Way. Mesquite grilled Steaks, Game, and other New West selections, all served in our authentic lodge. Enjoy our extensive collection of scotch, bourbon and draft beer & extensive wine selection. 862 W. BROADWAY • 733-3287 STEAK & GAME HOUSE 2 JACKSON HOLE SKIER 2009 CUSTOM JEWELRY BY JETER CASE In the log cabin next to Teton Theatre just off the town square 132 N. Cache Jackson Hole, Wyoming 307-733-5933 www.jcjewelers.com 800-358-5715 www. focusproductions. com 2009 JACKSON HOLE SKIER 3 H SKI • BIKE • BOARD • HIKE “The Locals’ Shop” SKIS, BOOTS, 520 W. BROADWAY & BINDINGS: OPEN DAILY Volkl • Rossignol 733-5335 r e • g Dalbello Tecnica e L On Broadway f f e J • • : Marker Salomon Full Tilt across from r e i k Bubba’s S s n o i SNOWBOARDS: t c u d o r Burton • Arbor • Prior P s u c o F Nitro • Salomon• Vans / y o K c M e CLOTHING: d a W : o • • t Obermeyer Orage Bonfire o h P Sherpa • Burton • Oakley www.hobacksports.com DEMOS A RENTALS RENTALSA REPAIR A SKIS A SNOWBOARDSA OUTWEAR 4 JACKSON HOLE SKIER 2009 32 years of inspiration at 6,000 feet Jewelry Originals Downtown Jackson 125 N.Cache, Gaslight Alley 307.733.2259 www.DanShelley.com [email protected] ALL DESIGNS COPYRIGHTED jackson hole skier 2008/2009 the resorts 24 Jackson Hole 34 The Tram Builders 38 Vertical-Foot Ski Records 40 Open-Boundary Protocol 41 Terrain Park & Superpipe 42 Snow King 46 Grand Targhee 52 White Pine the backcountry 12 Teton Skiing 54 Mountain Guides 64 Teton Pass Ambassador 66 Avalanche Education 68 Helicopter & Snowcat Skiing Skiers: Cover—Jason Tattersall Contents—Lynsey Dyer staff other stuff Publishers: Bob Woodall and Wade McKoy 8 Wildlife and National Parks d.b.a. -

Grand Teton National Park
GRAND TETON NATIONAL PARK • WTO MING * UNITED STATES DEPARTMENT OF THE INTERIOR NATIO NAL PARK SERVICE Grand Teton [WYOMING] National Park United States Department of the Interior Harold L. Ickes, Secretary NATIONAL PARK SERVICE Arno B. Cammerer, Director UNITED STATES GOVERNMENT PRINTING OFFICE WASHINGTON : 1936 Rules and Regulations -I-HE PARK regulations are designed for the protection of the natural Contents beauties as well as for the comfort and convenience of visitors. The follow ing synopsis is for the general guidance of visitors, who are requested to assist in the administration of the park by observing them. Copies of the complete rules and regulations promulgated by the Secretary of the Interior Page for the government of the park may be obtained at the office of the super History of the Region 3 intendent and at other points of concentration throughout the park. Geographic Features 7 The destruction, injury, defacement, or disturbance of any buildings, Teton Range 7 signs, equipment, trees, flowers, vegetation, rocks, minerals, animal, bird, Jackson Hole 9 or other life is prohibited. The Work of Glaciers 9 Camps must be kept clean. Rubbish and garbage should be burned. Trails 13 Refuse should be placed in cans provided for this purpose. If no cans are Mountain Climbing 14 provided where camp is made, refuse should be buried. Wildlife 18 Do not throw paper, lunch refuse, or other trash on the roads and trails. Trees and Plants 21 Carry until the same can be burned in camp or placed in receptacle. Naturalist Service 23 Fires shall be lighted only when necessary and when no longer needed Fishing 24 shall be completely extinguished. -
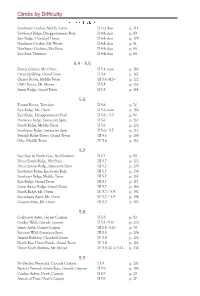
Teton-Rock-Climbs Climbs-By-Difficulty.Pdf
Climbs by Diffi culty 3rd - 4th class Southwest Couloir, Middle Teton II 3rd class p. 113 Southeast Ridge, Disappointment Peak II 4th class p. 89 East Ridge, Cloudveil Dome II 4th class p. 105 Northeast Couloir, Mt. Wister II 4th class p. 51 Northwest Couloirs, Nez Perce II 4th class p. 98 East Face, Teewinot II 4th class p. 68 5.4 - 5.5 Koven Couloir, Mt. Owen II 5.4 snow p. 184 Owen-Spalding, Grand Teton II 5.4 p. 142 Glacier Route, Middle Teton III 5.4 AI2+ p. 122 CMC Route, Mt. Moran II 5.5 p. 216 Exum Ridge, Grand Teton II 5.5 p. 144 5.6 Tunnel Route, Teewinot II 5.6 p. 70 East Ridge, Mt. Owen II 5.6 snow p. 186 East Ridge, Disappointment Peak II 5.6 / 5.7 p. 90 Durrance Ridge, Symmetry Spire II 5.6 p. 210 North Ridge, Middle Teton II 5.6 p. 126 Southwest Ridge, Symmetry Spire II 5.6 / 5.7 p. 212 Petzoldt Ridge Direct, Grand Teton III 5.6 p. 150 Dike, Middle Teton IV 5.6 p. 116 5.7 East Face & North Face, Red Sentinel II 5.7 p. 85 Direct South Ridge, Nez Perce III 5.7 p. 102 Direct Jensen Ridge, Symmetry Spire III 5.7 p. 208 Southwest Ridge, Enclosure Peak III 5.7 p. 134 Southeast Ridge, Middle Teton III 5.7 p. 114 East Ridge, Grand Teton III 5.7 p. 172 Lower Exum Ridge, Grand Teton III 5.7 p. 146 North Ridge, Mt. -

Grand Traverse
MOUNTAINMOUNTAIN COUNTRY COUNTRY Summer Guide to the Tetons & Yellowstone 2007 Vacation Adventures Boating • Hiking • Climbing Biking • Rodeo • Fishing Mountain Towns Area Map Wildlife FREE Contemporary outfitting women • men Trina Turk Paul & Joe Lacoste Mint Calypso Habitual True Religion Joe's Jeans Tory Burch Robert Graham Nanette Lepore Tocca 105 E. BROADWAY • 307-734-0067 4 MOUNTAIN COUNTRY 2007 64573 MRL Mtn Country Guide SirePage 1 4/11/07 1:30:15 PM Women’s Siren Ventilator Women’s-specific technology and styling for outdoor performance. Hoback Sports Jackson Bootlegger Teton Village Sports Siren selection varies by store. 520 W. Broadway Ave. 36 E. Broadway on the Square Teton Village 307.733.5335 307.733.6207 307.733.2181 Look for Merrell Apparel Fall 2007 EExplorexplore On the Cover: Bob Woodall photos: Mike Calabrese fishing Snake River; JH Rodeo; Snake River Canyon MOUNTAIN COUNTRY white water. Wade McKoy pho- tos: native cutthroat trout; Jason Tattersall bicycling Contents Photos: Clockwise from top left: white-water rafting the Snake; captive grizzly bear; calf roping at the rodeo; Western swing on the dance floor; mountain biking single- track; catching and releasing a cutthroat trout Publishers: Bob Woodall & Wade McKoy, Focus Productions, Inc. (FPI) Editors: Mike Calabrese, Wade McKoy, Bob Woodall Art Direction & Ad Design: Janet Melvin Advertising Sales: JACKSON HOLE & PINEDALE Kyli Fox, 307-733-6995 Photos: Wade McKoy, Bob Woodall, Grizzly and Wolf Discovery Center Grizzly and Wolf Bob Woodall, McKoy, Photos: Wade WEST YELLOWSTONE NATURE RECREATION Janet Melvin, 406-556-8655 CODY & DUBOIS 8 America’s National Parks 16 Hiking Bob Woodall, 307-733-6995 11 Issues of the Greater Yellowstone 18 Mountain Biking 14 Comfort & Safety in the Outdoors 21 Community Pathways Copyright 2007 by FPI (Focus Productions, Inc)., P.O. -

DBR Y W OREGON STATE
The Distribution and Biology of the A. 15 Oregon Trichoptera PEE .1l(-.", DBR Y w OREGON STATE Technical Bulletin 134 AGRICULTURAL 11 EXPERIMENTI STATION Oregon State University Corvallis, Oregon INovember 1976 FOREWORD There are four major groups of insectswhoseimmature stages are almost all aquatic: the caddisflies (Trichoptera), the dragonflies and damselflies (Odonata), the mayflies (Ephemeroptera), and the stoneflies (Plecoptera). These groups are conspicuous and important elements in most freshwater habitats. There are about 7,000 described species of caddisflies known from the world, and about 1,200 of these are found in America north of Mexico. All play a significant ro'e in various aquatic ecosystems, some as carnivores and others as consumers of plant tissues. The latter group of species is an important converter of plant to animal biomass. Both groups provide food for fish, not only in larval but in pupal and adult stages as well. Experienced fishermen have long imitated these larvae and adults with a wide variety of flies and other artificial lures. It is not surprising, then, that the caddisflies have been studied in detail in many parts of the world, and Oregon, with its wide variety of aquatic habitats, is no exception. Any significant accumulation of these insects, including their various develop- mental stages (egg, larva, pupa, adult) requires the combined efforts of many people. Some collect, some describe new species or various life stages, and others concentrate on studying and describing the habits of one or more species. Gradually, a body of information accumulates about a group of insects for a particular region, but this information is often widely scattered and much effort is required to synthesize and collate the knowledge. -

Species Risk Assessment
Ecological Sustainability Analysis of the Kaibab National Forest: Species Diversity Report Ver. 1.2 Prepared by: Mikele Painter and Valerie Stein Foster Kaibab National Forest For: Kaibab National Forest Plan Revision Analysis 22 December 2008 SpeciesDiversity-Report-ver-1.2.doc 22 December 2008 Table of Contents Table of Contents............................................................................................................................. i Introduction..................................................................................................................................... 1 PART I: Species Diversity.............................................................................................................. 1 Species List ................................................................................................................................. 1 Criteria .................................................................................................................................... 2 Assessment Sources................................................................................................................ 3 Screening Results.................................................................................................................... 4 Habitat Associations and Initial Species Groups........................................................................ 8 Species associated with ecosystem diversity characteristics of terrestrial vegetation or aquatic systems ...................................................................................................................... -

Grand Teton National Park Jackson District
National Park Service Craighead Beringia South Wyoming Game & Fish U.S. Department of the Interior State of Wyoming Grand Teton National Park Jackson District Howard Quigley Jackie Skaggs Mark Gocke 307-732-0188 307.739.3393 307.733.2383 ext 231 Interagency News Release For Immediate Release January 30, 2008 JACKSON- An ongoing mountain lion research project suffered a setback last Friday, January 25, when a lion kitten was killed during a routine capture operation in Grand Teton National Park. A local veterinarian and biologists from Craighead Beringia South, the Wyoming Game and Fish Department, and Grand Teton National Park were attempting to capture the 7-month old kitten of a radio-collared adult female mountain lion, when tracking hounds caught and killed the young cougar. Lion researchers routinely use hounds to safely track and tree the cats so that they can be tranquilized, collared and released. Unfortunately, in this case the kitten involved was in such poor health that it was unable to climb a tree and escape the dogs. “We‟re obviously devastated by the loss of the kitten,” said Teton Cougar Project leader, Howard Quigley. “We have captured a number of cats this age, and even younger, and they‟ve always treed well ahead of the dogs. This kitten was so emaciated it likely couldn‟t climb a tree. There were dozens available.” A necropsy performed by the crew‟s veterinarian, reported „very little body fat‟ and „marked muscle atrophy.‟ Officials believed the young cat probably would not have survived the winter. The kitten‟s mother, known as F101, has been the most productive breeding female in the history of the project, but is now old and possibly quickly losing her ability to provide for her young.