Assessment Report: the Clinical Effectiveness and Cost-Effectiveness
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent No.: US 9,381,189 B2 Green Et Al
US009381189B2 (12) United States Patent (10) Patent No.: US 9,381,189 B2 Green et al. (45) Date of Patent: Jul. 5, 2016 (54) INGREDIENTS FOR INHALATION AND (56) References Cited METHODS FOR MAKING THE SAME U.S. PATENT DOCUMENTS (75) Inventors: Matthew Michael James Green, 4,582,265 A * 4/1986 Petronelli ....................... 241.95 Wiltshire (GB); Richard Michael Poole, 6,257,233 B1 7/2001 Burr et al. 2004/01 18007 A1* 6/2004 Chickering et al. ............ 34/360 Wiltshire (GB) 2006, O257491 A1* 11, 2006 Morton et al. ... 424/489 (73) Assignee: VECTURA LIMITED, Wiltshire (GB) 2008/0063719 A1 3/2008 Morton et al. ................ 424/489 (*) Notice: Subject to any disclaimer, the term of this FOREIGN PATENT DOCUMENTS patent is extended or adjusted under 35 EP O709086 A2 5, 1996 U.S.C. 154(b) by 641 days. EP 14981 16 A1 1, 2005 GB 2387781 A 10, 2003 JP 2005298.347 10/2005 (21) Appl. No.: 13/514,672 JP 200954.1393 11, 2009 JP 2012,542618 6, 2012 (22) PCT Fled: Dec. 8, 2010 WO 96.23485 A1 8, 1996 WO 9703649 A1 2, 1997 (86) PCT NO.: PCT/GB2O10/052053 WO O2OO197 A1 1, 2002 WO O243701 A2 6, 2002 S371 (c)(1), WO 2005105043 A2 11/2005 Aug. 20, 2012 WO 2007053904 A1 5/2007 (2), (4) Date: WO 2008.000482 1, 2008 (87) PCT Pub. No.: WO2O11AO70361 WO 2009095684 A1 8, 2009 OTHER PUBLICATIONS PCT Pub. Date: Jun. 16, 2011 Brunauer et al. "Adsorption of Gases in Multimolecular Layers'. J. (65) Prior Publication Data Am. -
ANNNNNNNNNNNNNNNNNNNN 100A 006 Left Eye Input Right Eye Input
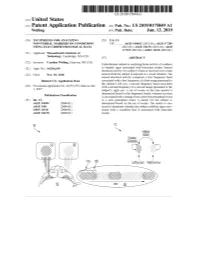
US 20190175049A1 ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2019 /0175049 A1 Welling ( 43 ) Pub . Date : Jun . 13 , 2019 ( 54 ) TECHNIQUES FOR ANALYZING (52 ) U . S . CI. NON -VERBAL MARKERS OF CONDITIONS CPC . .. A61B 5 /04842 (2013 . 01 ) ; A61B 5 / 7289 USING ELECTROPHYSIOLOGICAL DATA (2013 . 01) ; A61B 5 /0478 ( 2013 .01 ) ; A61B 5 /7225 ( 2013. 01 ) ; G06N 20 / 10 (2019 .01 ) (71 ) Applicant: Massachusetts Institute of Technology , Cambridge , MA (US ) ( 57 ) ABSTRACT (72 ) Inventor : Caroline Welling, Hanover, NH (US ) Embodiments related to analyzing brain activity of a subject to identify signs associated with binocular rivalry . Sensed ( 21 ) Appl. No. : 16 / 206, 639 electrical activity of a subject' s brain is received over a time period while the subject is exposed to a visual stimulus. The ( 22 ) Filed : Nov. 30 , 2018 sensed electrical activity comprises a first frequency band Related U . S . Application Data associated with a first frequency of a first image presented to the subject ' s left eye , a second frequency band associated (60 ) Provisional application No .62 / 593 , 535, filed on Dec . with a second frequency of a second image presented to the 1 , 2017 subject ' s right eye . A set of events in the time period is determined based on the frequency bands, wherein an event Publication Classification is associated with a change from a previous perceptual event (51 ) Int. Ci. to a new perceptual event. A metric for the subject is A61B 5 /0484 ( 2006 .01 ) determined based on the set of events . The metric is ana A61B 5 /00 ( 2006 .01 ) lyzed to determine whether the subject exhibits signs asso GO6N 20 / 10 (2006 .01 ) ciated with a condition that is associated with binocular A61B 5 /0478 ( 2006 .01 ) rivalry . -

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

Calcium Channel Blocker As a Drug Candidate for the Treatment of Generalised Epilepsies
UNIVERSITAT DE BARCELONA Faculty of Pharmacy and Food Sciences Calcium channel blocker as a drug candidate for the treatment of generalised epilepsies Final degree project Author: Janire Sanz Sevilla Bachelor's degree in Pharmacy Primary field: Organic Chemistry, Pharmacology and Therapeutics Secondary field: Physiology, Pathophysiology and Molecular Biology March 2019 This work is licensed under a Creative Commons license ABBREVIATIONS AED antiepileptic drug AMPA α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid ANNA-1 antineuronal nuclear antibody 1 BBB blood-brain barrier Bn benzyl BnBr benzyl bromide BnNCO benzyl isocyanate Boc tert-butoxycarbonyl Bu4NBr tetrabutylammonium bromide Ca+2 calcium ion CACNA1 calcium channel voltage-dependent gene cAMP cyclic adenosine monophosphate CCB calcium channel blocker cGMP cyclic guanosine monophosphate CH3CN acetonitrile Cl- chlorine ion Cmax maximum concentration CMV cytomegalovirus CTScan computed axial tomography DCM dichloromethane DIPEA N,N-diisopropylethylamine DMF dimethylformamide DMPK drug metabolism and pharmacokinetics DNET dysembryoplastic neuroepithelial tumours EEG electroencephalogram EPSP excitatory post-synaptic potential FDA food and drug administration Fe iron FLIPR fluorescence imaging plate reader fMRI functional magnetic resonance imaging GABA γ-amino-α-hydroxybutyric acid GAD65 glutamic acid decarboxylase 65 GAERS generalised absence epilepsy rat of Strasbourg GluR5 kainate receptor GTC generalised tonic-clonic H+ hydrogen ion H2 hydrogen H2O dihydrogen dioxide (water) -

A Comparative Study of Progabide, Valproate, and Epilepsy
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.49.11.1251 on 1 November 1986. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1986;49:1251-1257 A comparative study of progabide, valproate, and placebo as add-on therapy in patients with refractory epilepsy P CRAWFORD, D CHADWICK From the Department ofNeurology, Walton Hospital, Liverpool, UK SUMMARY A three way single blind cross-over comparison of progabide, valproate and placebo, as adjunctive therapy, was undertaken in 64 patients with therapy-resistant partial and generalised seizures. The study was not completed because of the incidence of elevated hepatic enzymes on progabide. Analysis of efficacy showed progabide to be inferior to valproate against all seizure types, particularly against tonic-clonic seizures. Valproate was superior to placebo against all seizure types, partial and tonic-clonic seizures. Progabide did not differ significantly from placebo in any instance. In addition progabide caused elevation of hepatic enzymes which was symptomatic in one case, and was associated with an interaction with phenytoin symptoms which resulted in of guest. Protected by copyright. intoxication in some cases. Progabide is a pro-drug and a GABA agonist which treatment limb was of six months with a two week washout possesses anticonvulsant properties in a variety of and cross-over period between treatment phases. Patients experimental models of seizures and epilepsy.' with severe, partial or generalised epilepsies were eligible for Whether it possesses antiepileptic properties in man is admission to the study as long as they had a definite history controversial. A number of double blind of epilepsy confirmed by observation and EEG recording studies and suffered a minimum of one seizure per month during the against placebo have been reported, some of which six months prior to entry into the study. -

Late Shri Vishnu Waman Thakur Charitable Trust`S VIVA INSTITUTE of PHARMACY At: Shirgaon, Veer Sawarkar Road, Virar (E), Taluka: Vasai, Dist
Late Shri Vishnu Waman Thakur Charitable Trust`s VIVA INSTITUTE OF PHARMACY At: Shirgaon, Veer Sawarkar Road, Virar (E), Taluka: Vasai, Dist. Palghar-401305, Maharashtra. FINAL YEAR UNIVERSITY EXAMINATION 2019-2020 Final Year B.Pharm. Semester VIII SUBJECT-BPH_C_801_T-Pharmaceutical Chemistry III MULTIPLE CHOICE QUESTIONS: PRACTICE QUESTION BANK Q. 1 Which is the correct IUPAC name for the following structure? A] 5-chloro-2-(methylamino)-5-phenyl-3H-1,4-benzodiazepine B] 7-chloro-2-(methylamino)-5-pyridinyl-3H-1,4-benzodiazepine-4-oxide C] 7-chloro-2-(ethylamino)-5-phenyl-3H-1,5-benzodiazepine D] 7-chloro-2-(methylamino)-5-phenyl-3H-1,4-benzodiazepine-4-oxide Q. 2 Which of the following is long acting sedative hypnotic? A] Diazepam B] Alprazolam C] Temazepam D] Imipramine Q. 3 Name of oxide derivative used as sedative hypnotic is A] Diazepam B] Chlordiazepoxide C] Nitazepam D] Ramelteon Late Shri Vishnu Waman Thakur Charitable Trust`s VIVA INSTITUTE OF PHARMACY At: Shirgaon, Veer Sawarkar Road, Virar (E), Taluka: Vasai, Dist. Palghar-401305, Maharashtra. Q. 4 With respect to the following general structure which is the correct statement ? A] X must be electropositive substituent for optimum activity B] X must be aromatic ring for optimum activity C] X must be electronegative substituent for optimum activity D] X must be H for optimum activity Q. 5 Which is the incorrect statement with respect to structure given in Q. 4 A] Ring C is ortho substituted with electron withdrawing group for optimum activity B] Ring C when para substituted increases activity C] Ring C is diortho substituted with electron withdrawing group for optimum activity D] Ring C when para substituted decreases activity Q. -

Nomination Background: Ketamine Hydrochloride (CASRN: 1867-66-9)
KETAMINE Nomination TABLE OF CONTENTS Page 1.0 BASIS OF NOMINATION 1 2.0 BACKGROUND INFORMATION 1 3.0 CHEMICAL PROPERTIES 2 3.1 Chemical Identification 2 3.2 Physico-Chemical Properties 3 3.3 Purity and Commercial Availability 4 4.0 PRODUCTION PROCESSES AND ANALYSIS 6 5.0 PRODUCTION AND IMPORT VOLUMES 7 6.0 USES 7 7.0 ENVIRONMENTAL OCCURRENCE 7 8.0 HUMAN EXPOSURE 7 9.0 REGULATORY STATUS 7 10.0 CLINICAL PHARMACOLOGY 7 11.0 TOXICOLOGICAL DATA 13 11.1 General Toxicology 13 11.2 Neurotoxicology 14 12.0 CONCLUSIONS 15 APPENDIX A 17 APPENDIX B 23 APPENDIX C 27 1 KETAMINE 1.0 BASIS OF NOMINATION Ketamine, a noncompetitive NMDA receptor blocker, has been used extensively off - label as a pediatric anesthetic for surgical procedures in infants and toddlers. Recently, Olney and coworkers have demonstrated severe widespread apoptotic degeneration throughout the rapidly developing brain of the 7-day-old rat after ketamine administration. Recent research at FDA has confirmed and extended Olney’s observations. These findings are cause for concern with respect to ketamine use in children. The issue of whether the neurotoxicity found in this animal model (rat) has scientific and regulatory relevance for the pediatric use of ketamine relies heavily upon confirmation of these findings that may be obtained from the conduct of an appropriate study in non-human primates. 2.0 BACKGROUND INFORMATION The issue of potential ketamine neurotoxicity in children surfaced as a result of FDA’s reluctance to approve an NIH pediatric clinical trial using this compound because of its documented neurotoxic effects in young rats (published in several papers over the last ten years by Olney and co-workers). -

Pharmaceutical Chemistry III MULTIPLE CHOICE QUESTIONS: PRACTICE QUESTION BANK
FINAL YEAR UNIVERSITY EXAMINATION 2019-2020 Final Year B.Pharm. Semester VIII SUBJECT-BPH_C_801_T-Pharmaceutical Chemistry III MULTIPLE CHOICE QUESTIONS: PRACTICE QUESTION BANK SET-I Q. 1 Which is the correct IUPAC name for the following structure? A] 5-chloro-2-(methylamino)-5-phenyl-3H-1,4-benzodiazepine B] 7-chloro-2-(methylamino)-5-pyridinyl-3H-1,4-benzodiazepine-4-oxide C] 7-chloro-2-(ethylamino)-5-phenyl-3H-1,5-benzodiazepine D] 7-chloro-2-(methylamino)-5-phenyl-3H-1,4-benzodiazepine-4-oxide Q. 2 Which of the following is long acting sedative hypnotic? A] Diazepam B] Alprazolam C] Temazepam D] Imipramine Q. 3 Name of oxide derivative used as sedative hypnotic is A] Diazepam B] Chlordiazepoxide C]Nitazepam D] Ramelteon Q. 4 With respect to the following general structure which is the correctstatement ? A] X must be electropositive substituent for optimum activity B] X must be aromatic ring for optimum activity C] X must be electronegative substituent for optimum activity D] X must be H for optimum activity Q. 5 Which is the incorrect statement with respect to structure given in Q. 4 A] Ring C is ortho substituted with electron withdrawing group for optimum activity B] Ring C when para substituted increases activity C] Ring C is diortho substituted with electron withdrawing group for optimum activity D] Ring C when para substituted decreases activity Q. 6 What is the starting material for synthesis of Piroxicam (structure given below) A] B] C] D] Q. 7 Which one of the following is Cytokine inhibitor? A] Abatacept B] Fluoxetine C] Propranolol D]Aldosterone Q. -

The Effects of Ketamine on Dopaminergic Function
THE EFFECTS OF KETAMINE ON DOPAMINERGIC FUNCTION Michelle Kokkinou Psychiatric Imaging Group London Institute of Medical Sciences (LMS), Medical Research Council (MRC) Faculty of Medicine, Imperial College London Thesis submitted for the degree of Doctor of Philosophy 1 Declaration of originality The experiments described and data analysis were part of my own work, unless otherwise stated. The work was conducted at the London Institute of Medical Sciences (LMS) in collaboration with Imanova Ltd. Specialist pre-clinical imaging analysis was conducted in collaboration with Mattia Veronese (King’s College London). Specialist meta-analysis was conducted in collaboration with Abhishekh Ashok. Peer-reviewed accepted paper Michelle Kokkinou, Abhishekh H Ashok, Oliver D Howes. The effects of ketamine on dopaminergic function: meta-analysis and review of the implications for neuropsychiatric disorders. Molecular Psychiatry. Oct 3. doi: 10.1038/mp.2017.190. Published conference abstracts arising from this thesis Chapters 3&4: Howes OD., Kokkinou M. The Effects of Repeated NMDA Blockade with Ketamine on Dopamine and Glutamate Function. Biological Psychiatry. 72nd Annual Scientific Convention and Meeting. https://doi.org/10.1016/j.biopsych.2017.02.091 Chapters 4&5: Kokkinou M, Irvine EE, Bonsall DR Ungless MA, Withers DJ, Howes OD. Modulation of sub-chronic ketamine-induced locomotor sensitisation by midbrain dopamine neuron firing. European Neuropsychopharmacology (ENP) Chapters 3 & 4 are in preparation for publication. Presentation regarding the experiments in this thesis British Association for Psychopharmacology Meeting July 2017. Midbrain dopamine neuron firing mediates the effects of ketamine treatment on locomotor activity: A DREADD experiment 2 Copyright Declaration The copyright of this thesis rests with the author and is made available under a Creative Commons Attribution Non-Commercial No Derivatives Licence. -

General Agreement on Tariffs Andtrade
RESTRICTED GENERAL AGREEMENT TAR/W/87/Rev.1 16 June 1994 ON TARIFFS AND TRADE Limited Distribution (94-1266) Committee on Tariff Concessions HARMONIZED COMMODITY DESCRIPTION AND CODING SYSTEM (Harmonized System) Classification of INN Substances Revision The following communication has been received from the Nomenclature and Classification Directorate of the Customs Co-operation Council in Brussels. On 25 May 1993, we sent you a list of the INN substances whose classification had been discussed and decided by the Harmonized System Committee. At the time, we informed you that the classification of two substances, clobenoside and meclofenoxate, would be decided later. Furthermore, for some of the chemicals given in that list, one of the contracting parties had entered a reservation and the Harmonized System Committee therefore reconsidered its earlier decision in those cases. I am therefore sending you herewith a revised complete list of the classification decisions of the INN substances. In this revised list, two substances have been added and the classifications of two have been revised as explained below: (a) Addition Classification of clobenoside, (subheading 2940.00) and meclofenoxate (subheading 2922.19). (b) Amendment Etafedrine and moxidentin have now been reclassified in subheadings 2939.40 and 2932.29 respectively. The list of INN substances reproduced hereafter is available only in English. TAR/W/87/Rev. 1 Page 2 Classification of INN Substances Agreed by the Harmonized System Committee in April 1993 Revision Description HS Code -

Driver Impairment, Accident Risk and Tolerance
IMMORTAL D-R4.4 21/10/04 Deliverable D-R4.4 EXPERIMENTAL STUDIES ON THE EFFECTS OF LICIT AND ILLICIT DRUGS ON DRIVING PERFORMANCE, PSYCHOMOTOR SKILLS AND COGNITIVE FUNCTION Public IMMORTAL CONTRACT NO GMAI-2000-27043 SI2.319837 Project Co-ordinator: Prof. Bob Hockey, University of Leeds Workpackage Leader: Inger Marie Bernhoft, Danish Transport Research Institute Authors: JG Ramaekers, KPC Kuypers, Brain & Behavior Institute, Faculty of Psychology, Maastricht University CM Wood, GRJ Hockey, S Jamson, H Jamson, E Birch, School of Psychology, University of Leeds Date: 28.09.2004 Project Funded by the European Commission under the Transport RTD Programme of the 5th Framework Programme i IMMORTAL D-R4.4 21/10/04 School of Psychology, UNIV LEEDS DOCUMENT CONTROL INFORMATION Title Experimental studies on the effects of licit and illicit drugs on driving performance, psychomotor skills and cognition Author(s) JG Ramaekers, KPC Kuypers, CM Wood, GRJ Hockey, S Jamson, H Jamson, E Birch Editor(s) Date 28/09/2004 Report Number Reference and version Version 1 number Distribution Project Consortium Availability Public File QA check Nic Ward Authorised by Bob Hockey Signature ii IMMORTAL D-R4.4 21/10/04 Table of Contents 1. INTRODUCTION.............................................................................................................10 1.1 GENERAL BACKGROUND....................................................................................10 1.2 TASK DESCRIPTION.............................................................................................11 -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational