Annex Ii Asean Guiding Principles for the Use of Additives and Excipients in Health Supplements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Morpholine: a Glazing Agent for Fruits and Vegetables Coating/Waxing (IJSTE/ Volume 2 / Issue 11 / 119) with Glazing Agent
IJSTE - International Journal of Science Technology & Engineering | Volume 2 | Issue 11 | May 2016 ISSN (online): 2349-784X Morpholine: A Glazing Agent for Fruits and Vegetables Coating/Waxing Rupak Kumar Suman Kapur Research Scholar Senior Professor Department of Biological Sciences Department of Biological Sciences BITS-Pilani, Hyderabad Campus, Hyderabad-500078, India BITS-Pilani, Hyderabad Campus, Hyderabad-500078, India Abstract The saying “an apple a day keeps the doctor away” probably gives us the impression that apples are the healthiest fruits. But besides the fact that it rhymes, does it really have no adverse effects if we eat a bright red wax coated apple every day? Morpholine (C4H9NO) is a chemical used as emulsifier in the preparation of wax coatings for fruits and vegetables to help them last longer and remain fresh even during prolonged transit. Morpholine oleate is added to wax as it enables spreading wax in water based liquid for use as a protective coating to prevent contamination by pests and diseases. Morpholine alone does not appear to pose a health concern because morpholine itself is neither a carcinogen nor a teratogen and does not cause chronic toxicity. However, it is a precursor for potent carcinogenic nitrosamines. Keywords: Carcinogen, Emulsifier, Morpholine, Teratogen, Wax Coating ________________________________________________________________________________________________________ I. INTRODUCTION The practice of fruit/vegetable coating was accepted long before their associated chemistries were understood, and are still practiced till date. The first wax coating was applied to citrus fruits in 12th-13th centuries in China. Today, it has expanded rapidly for retaining quality of a wide variety of foods/vegetables, with total annual revenue exceeding $100 million [1]. -

Rosin - Wikipedia, the Free Encyclopedia Page 1
Rosin - Wikipedia, the free encyclopedia Page 1 Rosin From Wikipedia, the free encyclopedia Rosin , also called colophony or Greek pitch (Pix græca ), is a solid form of resin obtained from pines and some other plants, mostly conifers, produced by heating fresh liquid resin to vaporize the volatile liquid terpene components. It is semi-transparent and varies in color from yellow to black. At room temperature rosin is brittle, but it melts at stove-top temperatures. It chiefly consists of various resin acids, especially abietic acid.[1] The term "colophony" comes from colophonia resina or "resin from the pine trees of Colophon," an ancient Ionic city. A cake of rosin, made for use by violinists, used here for Contents soldering 1 Uses 1.1 Pharmaceutical 2 Production 3 Properties 4 Sources 5 See also 6 Sources 7 Notes 8 External links Uses Rosin is an ingredient in printing inks, photocopying and laser printing paper, varnishes, adhesives (glues), soap, paper sizing, soda, soldering fluxes, and sealing wax. Rosin can be used as a glazing agent in medicines and chewing gum. It is denoted by E number E915. A related glycerol ester (E445) can be used as an emulsifier in soft drinks. In pharmaceuticals, rosin forms an ingredient in several plasters and ointments. In industry, rosin is a flux used in soldering. The lead-tin solder commonly used in electronics has about 1% rosin as a flux core helping the molten metal flow and making a better connection by reducing the refractory solid oxide layer formed at the surface back to metal. It is frequently seen as the burnt or clear residue around new soldering. -

Chemical and Technical Assessment 65Th JECFA
Chemical and Technical Assessment 65th JECFA BEESWAX Chemical and Technical Assessment (CTA) First draft prepared by Paul M. Kuznesof, Ph.D.∗ Reviewed by D. Brian Whitehouse, Ph.D. 1 Summary Beeswax (INS No. 901) consists primarily of a mixture of esters of fatty acids and fatty alcohols, paraffinic hydrocarbons, and free fatty acids; minor amounts of free fatty alcohols are also present. Two types of beeswax are marketed: yellow beeswax (C.A.S No. 8006-40-4) and white beeswax (C.A.S. No. 8012-89-3). Yellow beeswax is a yellow or light-brown solid that is somewhat brittle when cold and presents a characteristic odour of honey. White beeswax is a white or yellowish white solid (thin layers are translucent) having a characteristic, but faint, odour of honey. Beeswax is obtained from the honeycombs of bees (Apis mellifera L., Fam. Apidae) after removal of the honey. The combs are melted with hot water, steam, or solar heat. After removing the insoluble impurities, the liquid wax is cast into cakes for further purification to obtain food-grade yellow beeswax. Bleaching the latter with e.g. hydrogen peroxide, sulfuric acid or sunlight, yields white beeswax. Beeswax consists primarily of five main groups of components, namely: 1. Free fatty acids (typically 12-14%), most of which are saturated (ca. 85%) and have a chain length of C24-C32. 2. Free primary fatty alcohols (ca. 1%) with a chain length of C28-C35. 3. Linear wax monoesters and hydroxymonoesters (35-45%) with chain lengths generally of C40- C48. The esters are derived almost exclusively from palmitic acid, 15-hydroxypalmitic acid, and oleic acid. -

Decco Communication on Shellac Resin
Decco Communication on Shellac Resin 1 BACKGROUND Some supermarkets in Australia have inquired about the quality of shellac resin used as an ingredient in apple wax coatings. Apple packers, who are EE Muir’s customers, have in turn asked EE Muir to provide documentation on the quality of shellac and manufacturing practices used by Decco. Below are a number of documents and explanations that Decco is providing to EE Muir in support of Decco’s quality and manufacturing practices. 2 WHAT IS NATURAL SHELLAC RESIN Shellac is a natural gum resin secreted by the lac beetle (laccifer lacca) which lives on trees in tropical and subtropical regions. The natural gum resin is harvested from the trees and then processed and refined into flakes and powders of pure natural shellac resin. 3 SHELLAC AND ITS USES Natural shellac resin has unique properties and is used in various applications such as: Fruit coatings, Confectionery (Candy & Chocolates coating), Pharmaceutical (Tablets coatings) as well as several non- food segments. Shellac is used as a 'wax' coating on citrus fruit to prolong its shelf/storage life. It is also used to replace the natural wax of the apple, which is removed during the cleaning & washing process. In these applications, the wax coating prevents moisture loss (shrinking, shriveling of the fruit) and preserves the textural and eating quality of the fruit. When used for this purpose, it has the food additive E number E904 which is recognized and adopted worldwide. Shellac is also used as a glazing agent on pills, food, chocolate & candies, in the form of pharmaceutical glaze or, confectioner's glaze. -

E Number from Wikipedia, the Free Encyclopedia
E number From Wikipedia, the free encyclopedia E numbers are codes for substances which can be used as food additives for use within the European Union[1] and Switzerland (the "E" stands for "Europe").[2] They are commonly found on food labels throughout the European Union.[3] Safety assessment and approval are the responsibility of the European Food Safety Authority.[4] Having a single unified list for food additives was first agreed upon in 1962 with colours. In 1964, the directives for preservatives were added, 1970 for antioxidants and 1974 for the emulsifiers, stabilisers, thickeners and gelling agents.[5] Contents A solution of E101 riboflavin (also 1 Numbering scheme known as Vitamin B2) 2 Colloquial use 3 Classification by numeric range 4 Full list 4.1 E100–E199 (colours) 4.2 E200–E299 (preservatives) 4.3 E300–E399 (antioxidants, acidity regulators) 4.4 E400–E499 (thickeners, stabilizers, emulsifiers) 4.5 E500–E599 (acidity regulators, anti-caking Crystals of E621 Monosodium glutamate, a flavour enhancer agents) 4.6 E600–E699 (flavour enhancers) 4.7 E700–E799 (antibiotics) 4.8 E900–E999 (glazing agents and sweeteners) 4.9 E1000–E1599 (additional chemicals) 5 See also 6 Notes 7 External links Numbering scheme The numbering scheme follows that of the International Numbering System (INS) as determined by the Codex Alimentarius committee,[6] though only a subset of the INS additives are approved for use in the European Union as food additives. E numbers are also encountered on food labelling in other jurisdictions, including the Cooperation Council for the Arab States of the Gulf, Australia, New Zealand[7] and Israel. -

Gourmet Gummy Candy from Germany
Gourmet Gummy Candy from Germany Fruity-Sin is a German and American Gummy Candy company based Germany Our U.S. office is in Miami, Florida. Our Gourmet Gummies are produced in Germany. Delivery is made from Germany or Miami. Why is "Fruity-Sin" the perfect source for your sweet needs? When we say "Gourmet Gummy Candy", then we mean GOURMET. We know that we have outstanding QUALITY, this is the reason for buying from us. Why is our Gummy GOURMET? We use Real Fruit Juice. Some of them up to 50% We use Natural Fruit Sugar We use Natural Colors Some basic information: Quality Do you demand the BEST quality? We have it. Do you want to satisfy your customers? Us too. Price Quality never could be cheap, neither are we. But our product is not expensive if you consider the superior quality. Rebate We do not offer rebates, but the more you buy, the cheaper we sell. Ask for our quantity discount. MOQ Each flavor has a Minimum Order Quantity of 3.000 gram (6.6 lb) Delivery The delivery time is usually 3-4 weeks from Germany. If we have enough stock in Miami we will be much faster. Private Label We can produce any flavor, size, or shape of gourmet gummy candy. Just ask us. Shelf Life More than 12 months. Store in a cool, dry, and dark location for longest shelf life. Shelf life in this case up to 24 months. P-2 Prosecco Gold-Bears Glukosesirup, Zucker, Wasser, Gelatine, Säuerungsmittel: Citronensäure, Aroma, Farbstoffe: Echtes Karmin, Lutein, Überzugsmittel: Pflanzenöl (Kokosnuss, Raps), Carnaubawachs, 24k Gold E175 Lactose Free Gluten Free No artificial -

Application of Rice Bran Wax Organogel to Substitute Solid
APPLICATION OF RICE BRAN WAX ORGANOGEL TO SUBSTITUTE SOLID FAT AND ENHANCE UNSATURATED FAT CONTENT IN ICE CREAM by Daniele Cristina Zulim Botega A Thesis Presented to The University of Guelph In partial fulfillment of requirements for the degree of Master of Science in Food Science Guelph, Ontario, Canada © Daniele C. Zulim Botega, January, 2012 ABSTRACT APPLICATION OF RICE BRAN WAX ORGANOGEL TO SUBSTITUTE SOLID FAT AND ENHANCE UNSATURATED FAT CONTENT IN ICE CREAM Daniele C. Zulim Botega Advisor: Professor H. D. Goff, Dr. University of Guelph, 2012 Co-Advisor: Professor A. Marangoni, Dr. The objective of this study was to investigate the potential application of rice bran wax (RBW) organogel to replace solid fat content and create the fat network in ice cream. Ice creams with 10% fat or 15% fat were formulated with RBW organogel as the fat source, and two different emulsifiers were used: polmo, a commercial blend of emulsifiers which contains 80% mono- and diglycerides and 20% polysorbate 80, and Glycerol monooleate (GMO). Candelilla wax (CDW) organogel and carnauba wax (CBW) organogel were also tested for comparison. RBW organogel had the ability to form and sustain structure in 15% fat ice creams when GMO was used as the emulsifier. Transmission electron microscopy revealed that the RBW crystal morphology within the fat droplet, when GMO was used as the emulsifier, was characterized by the growth of crystals at the outer edge of the droplet which increased fat destabilization and network formation. ACKNOWLEDGEMENTS I would like to express my sincere appreciation to my advisor Dr. Douglas Goff for granting me the opportunity to undertake this intriguing project. -

Food Product Standards and Food Additives
163 Appendix A : List of Food Additives Use of Food Additives in Food Products: Food products may contain additives as specified in the Regulations and in the following tables Table 1 List of food additives for use in bread and biscuits Sl. No. Name of additive Bread Biscuits 12 3 4 A. Acidity regulators 1 Sodium fumarate GMP GMP 2 Potassium malate GMP GMP 3 Sodium hydroxide GMP GMP 4 Acetic acid or Lactic acid 2500 ppm max GMP 5 Citric acid - GMP 6 Malic acid - GMP 7 Tartaric acid - GMP B. Emulsifying and stabilizing agents singly or in - Emulsifying and stabilizing combination agents listed in regulation 3.1.6 suitable for this product may be used. 1 Sucroglycerides - 1000 ppm max 2 Hydroxy Propyl methyl cellulose GMP GMP 3 Sucrose esters of fatty acids GMP GMP 4 Di- Acetyl tartaric acid esters of mono and GMP 10000 ppm max di- glycerides 5 Guar gum 5000 ppm max — 6 Sorbitol GMP — 7 Lecithin GMP — 8 Glycerine GMP — 9 Glycerol monostearate GMP — 10 Sodium steroyl 2 lactylate of 5000 ppm max — Calcium steroyl 2 lactylate (Singly or in combination) 11 Polyglycerol esters of fatty acids and polyglycerol 2000 ppm max — esters of interesterified recinoleic acid C. Improver 1 Fungal alpha amylase 100 ppm max (on flour — mass basis) 2 Bacterial amylase GMP GMP 3 Amylases and other enzymes - GMP 4 Ammonium persulphate 2500 ppm max (on flour — mass basis) 5 Calcium phosphate 2500 ppm max (on flour — mass basis) 6 Calcium carbonate 5000 ppm max (on flour — mass basis) 164 12 3 4 7 Potassium bromate and/or Potassium iodate 50 ppm max (On flour — mass basis) D. -

Draft Revision of Class Names and the International
CLASS NAMES AND THE INTERNATIONAL NUMBERING SYSTEM FOR FOOD ADDITIVES CAC/GL 36-1989 Adopted in 1989. Revision: 2008. Amendment: 2015. Section 3 and 4 “International Numbering System for Food Additives” are regularly updated. CAC/GL 36-1989 2 SECTION 1 - INTRODUCTION BACKGROUND The International Numbering System for Food Additives (INS) is intended as a harmonised naming system for food additives as an alternative to the use of the specific name, which may be lengthy. Inclusion in the INS does not imply approval by Codex for use as food additives. The list may include those additives that have not been evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA). The INS does not include flavourings, which have a JECFA number as identifier, chewing gum bases, and dietetic and nutritive additives. Enzymes which function as food additives have been included in an 1100 series. EXPLANATORY NOTES ON THE LAY-OUT OF THE INS The INS in numerical order (Section 3) is set out in four columns giving the identification number, the name of the food additive, the functional classes and the technological purposes. The identification number usually consists of three or four digits such as 100 for curcumins and 1001 for choline salts and esters. However, in some instances the number is followed by an alphabetical suffix, for example, 150a identifies Caramel I – plain caramel and 150b identifies Caramel II - sulfite caramel. The alphabetical designations are included in order to further characterize the different classes of additives (e.g. caramel produced by different processes). Under the column listing the name of the food additive, some additives are further subdivided by numerical subscripts. -
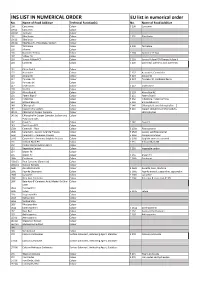
INS LIST in NUMERICAL ORDER EU List in Numerical Order No
INS LIST IN NUMERICAL ORDER EU list in numerical order No. Name of Food Additive Technical Function(s) No. -

Technical Evaluation Report – Carnauba Wax (2014)
Carnauba Wax Handling/Processing 1 2 Identification of Petitioned Substance 11 Carnauba Wax, Carnauba Wax Type 1, Type 3 or 3 Chemical Names: 12 Type 4 Flakes, Powder or Lumps 4 Carnauba wax 5 CAS Numbers: 6 Other Name: 8015-86-9 7 Brazil wax, palm wax, ceara wax; 8 INCI name: Copernicia cerifera (carnauba) wax Other Codes: 9 INS number 903, E number E903, EC Number 10 Trade Names: 232-399-4 13 14 Summary of Petitioned Use 15 16 Carnauba wax is listed at 7 CFR Part 205.605(a) as one of two nonsynthetic waxes allowed as ingredients 17 in or on processed products labeled as “organic” or “made with organic (specified ingredients or food 18 group(s)).” There are no other specific restrictions on how carnauba wax may be used in the organic 19 regulations. 20 21 Characterization of Petitioned Substance 22 23 Composition of the Substance: 24 Carnauba wax is an amorphous, complex mixture of several compounds, predominantly esters such as 25 aliphatic esters, alpha-hydroxy esters and cinnamic aliphatic diesters. It also contains free acids, free 26 alcohols, hydrocarbons and resins (European Food Safety Authority 2012) (Joint FAO/WHO Expert 27 Committee on Food Additives 2006). It has anywhere from 0.5 -11% water (Bennett 1963). In its raw form, 28 carnauba wax contains impurities in the form of unsaponifiable residues originating from the carnauba 29 palm plant ( iniste rio da Agricultura, Pecuaria e Abastecimento, Gabinete de Ministro 2004). These 30 comprise 50-55% of raw carnauba wax (Joint FAO/WHO Expert Committee on Food Additives 2006). -

Warta Kerajaan S E R I P a D U K a B a G I N D a DITERBITKAN DENGAN KUASA
M A L A Y S I A Warta Kerajaan S E R I P A D U K A B A G I N D A DITERBITKAN DENGAN KUASA HIS MAJESTY’S GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY Jil. 61 TAMBAHAN No. 7 6hb April 2017 TMA No. 17 No. TMA 58. AKTA CAP DAGANGAN 1976 (Akta 175) PENGIKLANAN PERMOHONAN UNTUK MENDAFTARKAN CAP DAGANGAN Menurut seksyen 27 Akta Cap Dagangan 1976, permohonan-permohonan untuk mendaftarkan cap dagangan yang berikut telah disetuju terima dan adalah dengan ini diiklankan. Jika sesuatu permohonan untuk mendaftarkan disetuju terima dengan tertakluk kepada apa-apa syarat, pindaan, ubahsuaian atau batasan, syarat, pindaan, ubahsuaian atau batasan tersebut hendaklah dinyatakan dalam iklan. Jika sesuatu permohonan untuk mendaftarkan di bawah perenggan 10(1)(e) Akta diiklankan sebelum penyetujuterimaan menurut subseksyen 27(2) Akta itu, perkataan-perkataan “Permohonan di bawah perenggan 10(1)(e) yang diiklankan sebelum penyetujuterimaan menurut subseksyen 27(2)” hendaklah dinyatakan dalam iklan itu. Jika keizinan bertulis kepada pendaftaran yang dicadangkan daripada tuanpunya berdaftar cap dagangan yang lain atau daripada pemohon yang lain telah diserahkan, perkataan-perkataan “Dengan Keizinan” hendaklah dinyatakan dalam iklan, menurut peraturan 33(3). WARTA KERAJAAN PERSEKUTUAN WARTA KERAJAAN PERSEKUTUAN 4010 [6hb April 2017 6hb April 2017] PB Notis bangkangan terhadap sesuatu permohonan untuk mendaftarkan suatu cap dagangan boleh diserahkan, melainkan jika dilanjutkan atas budi bicara Pendaftar, dalam tempoh dua bulan dari tarikh Warta ini, menggunakan Borang CD 7 berserta fi yang ditetapkan. TRADE MARKS ACT 1976 (Act 175) ADVERTISEMENT OF APPLICATION FOR REGISTRATION OF TRADE MARKS Pursuant to section 27 of the Trade Marks Act 1976, the following applications for registration of trade marks have been accepted and are hereby advertised.