Chemical and Technical Assessment 65Th JECFA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Morpholine: a Glazing Agent for Fruits and Vegetables Coating/Waxing (IJSTE/ Volume 2 / Issue 11 / 119) with Glazing Agent
IJSTE - International Journal of Science Technology & Engineering | Volume 2 | Issue 11 | May 2016 ISSN (online): 2349-784X Morpholine: A Glazing Agent for Fruits and Vegetables Coating/Waxing Rupak Kumar Suman Kapur Research Scholar Senior Professor Department of Biological Sciences Department of Biological Sciences BITS-Pilani, Hyderabad Campus, Hyderabad-500078, India BITS-Pilani, Hyderabad Campus, Hyderabad-500078, India Abstract The saying “an apple a day keeps the doctor away” probably gives us the impression that apples are the healthiest fruits. But besides the fact that it rhymes, does it really have no adverse effects if we eat a bright red wax coated apple every day? Morpholine (C4H9NO) is a chemical used as emulsifier in the preparation of wax coatings for fruits and vegetables to help them last longer and remain fresh even during prolonged transit. Morpholine oleate is added to wax as it enables spreading wax in water based liquid for use as a protective coating to prevent contamination by pests and diseases. Morpholine alone does not appear to pose a health concern because morpholine itself is neither a carcinogen nor a teratogen and does not cause chronic toxicity. However, it is a precursor for potent carcinogenic nitrosamines. Keywords: Carcinogen, Emulsifier, Morpholine, Teratogen, Wax Coating ________________________________________________________________________________________________________ I. INTRODUCTION The practice of fruit/vegetable coating was accepted long before their associated chemistries were understood, and are still practiced till date. The first wax coating was applied to citrus fruits in 12th-13th centuries in China. Today, it has expanded rapidly for retaining quality of a wide variety of foods/vegetables, with total annual revenue exceeding $100 million [1]. -

Rosin - Wikipedia, the Free Encyclopedia Page 1
Rosin - Wikipedia, the free encyclopedia Page 1 Rosin From Wikipedia, the free encyclopedia Rosin , also called colophony or Greek pitch (Pix græca ), is a solid form of resin obtained from pines and some other plants, mostly conifers, produced by heating fresh liquid resin to vaporize the volatile liquid terpene components. It is semi-transparent and varies in color from yellow to black. At room temperature rosin is brittle, but it melts at stove-top temperatures. It chiefly consists of various resin acids, especially abietic acid.[1] The term "colophony" comes from colophonia resina or "resin from the pine trees of Colophon," an ancient Ionic city. A cake of rosin, made for use by violinists, used here for Contents soldering 1 Uses 1.1 Pharmaceutical 2 Production 3 Properties 4 Sources 5 See also 6 Sources 7 Notes 8 External links Uses Rosin is an ingredient in printing inks, photocopying and laser printing paper, varnishes, adhesives (glues), soap, paper sizing, soda, soldering fluxes, and sealing wax. Rosin can be used as a glazing agent in medicines and chewing gum. It is denoted by E number E915. A related glycerol ester (E445) can be used as an emulsifier in soft drinks. In pharmaceuticals, rosin forms an ingredient in several plasters and ointments. In industry, rosin is a flux used in soldering. The lead-tin solder commonly used in electronics has about 1% rosin as a flux core helping the molten metal flow and making a better connection by reducing the refractory solid oxide layer formed at the surface back to metal. It is frequently seen as the burnt or clear residue around new soldering. -
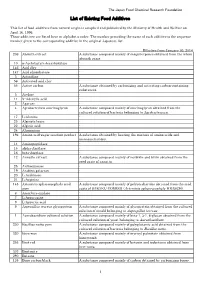
List of Existing Food Additives
The Japan Food Chemical Research Foundation List of Existing Food Additives This list of food additives from natural origin is complied and published by the Ministry of Health and Welfare on April 16, 1996. These additives are listed here in alphabetic order. The number preceding the name of each additive is the sequence number given to the corresponding additive in the original Japanese list. Effective from January 30, 2014 236 Absinth extract A substance composed mainly of sesquiterpenes obtained from the whole absinth grass. 10 α-Acetolactate decarboxylase - 146 Acid clay - 147 Acid phosphatase - 3 Actinidine - 56 Activated acid clay - 55 Active carbon A substance obtained by carbonizing and activating carbon-containing substances. 5 Acylase - 11 5'-Adenylic acid - 2 Agarase - 4 Agrobacterium succinoglycan A substance composed mainly of succinoglycan obtained from the cultured solution of bacteria belonging to Agrobacteriurn. 17 L-Alanine - 23 Alginate lyase - 22 Alginic acid - 24 Aluminium - 196 Amino acid-sugar reaction product A substance obtained by heating the mixture of amino acids and monosaccharides. 14 Aminopeptidase - 15 alpha-Amylase - 16 beta-Amylase - 12 Annatto extract A substance composed mainly of norbixin and bixin obtained from the seed coats of annatto. 25 Anthocyanase - 19 Arabino galactan - 20 L-Arabinose - 21 L-Arginine - 145 Artemisia sphaerocephala seed A substance composed mainly of polysaccharides obtained from the seed gum coats of SABAKU-YOMOGI (Artemisia sphaerocephala KRASCH). 6 Ascorbate oxidase - 7 L-Asparagine - 8 L-Aspartic acid - 9 Aspergillus terreus glycoprotein A substance composed mainly of glycoprotein obtained from the cultured solution of mould belonging to Aspergillus terreus . 1 Aureobasidium cultured solution A substance composed mainly of beta-1, 3-1, 6-glucan obtained from the cultured solution of yeast belonging to Aureobasidium . -

Sales Brochure Rev M 01A-8-15 FINAL BLU
Waxes Source Grades Colors Forms Uses & Special Features BEESWAX Honeycomb of the Crude, bleached, re- Cream-white to Cakes, slabs, pas- Color Cosmetics, Lip Care, Bee fined yellow, white, dark brown tilles, granular. Mascara, Eye Makeup, Hair cosmetic Wax, Creams, Lotions, Pharma- ceuticals, Ointments, Tablet Coatings, Confectionary Glazes, Candles. CANDELILLA Scales covering reed Crude, Refined, Light brown to Crude lumps, Color Cosmetics, Lip Care, like plants in Texas Bleached light yellow. refined lumps, Mascara, Eye Makeup, Hair and Mexico flakes, granular, & Wax, Pharmaceuticals, Tablet powder Coatings, Polishes, Precision Casting, Adhesives, Chewing Gum Base. CARNAUBA Exuded by the leaves No. 1 Yellow (Type 1) Yellow to Lumps, flakes, Color Cosmetics, Lip Care, of Brazilian "Tree of NC #3 Light (Type 3) brownish green. granular, & pow- Mascara, Eye Makeup, Pharma- Life" NC#3 Dark (Type 4- der ceuticals, Ointments, Tablet Filtered) Coatings, Confectionary Glazes, NC#3 Centrifuged Investment Casting, Polishes, , (Type 4) Inks and Thermal Transfer Rib- bon Coating, Fruit Wax Emul- sion. CERESINE & Formulated hydrocar- Specific to melting White Slabs, pastilles, & Color Cosmetics, Lip Care, OZOKERITE bons point, penetration, and granular. Mascara, Eye Makeup, Hair gel strength. Wax, Creams, Lotions, Pharma- ceuticals, Ointments, Coatings, Lubricants, Waterproofing, Adhesives. PARAFFIN & Petroleum Waxes Refined to Melt point White to Yellow Slabs, & pastilles. Cosmetics, Color Cosmetics, MICROCRYSTAL- & Color Lip Care, Mascara, Eye -

Decco Communication on Shellac Resin
Decco Communication on Shellac Resin 1 BACKGROUND Some supermarkets in Australia have inquired about the quality of shellac resin used as an ingredient in apple wax coatings. Apple packers, who are EE Muir’s customers, have in turn asked EE Muir to provide documentation on the quality of shellac and manufacturing practices used by Decco. Below are a number of documents and explanations that Decco is providing to EE Muir in support of Decco’s quality and manufacturing practices. 2 WHAT IS NATURAL SHELLAC RESIN Shellac is a natural gum resin secreted by the lac beetle (laccifer lacca) which lives on trees in tropical and subtropical regions. The natural gum resin is harvested from the trees and then processed and refined into flakes and powders of pure natural shellac resin. 3 SHELLAC AND ITS USES Natural shellac resin has unique properties and is used in various applications such as: Fruit coatings, Confectionery (Candy & Chocolates coating), Pharmaceutical (Tablets coatings) as well as several non- food segments. Shellac is used as a 'wax' coating on citrus fruit to prolong its shelf/storage life. It is also used to replace the natural wax of the apple, which is removed during the cleaning & washing process. In these applications, the wax coating prevents moisture loss (shrinking, shriveling of the fruit) and preserves the textural and eating quality of the fruit. When used for this purpose, it has the food additive E number E904 which is recognized and adopted worldwide. Shellac is also used as a glazing agent on pills, food, chocolate & candies, in the form of pharmaceutical glaze or, confectioner's glaze. -

Celebrating the Rich History of Waxes Bladel, the Netherlands What’S Inside: Watertown, Connecticut, Usa
CELEBRATING THE RICH HISTORY OF WAXES BLADEL, THE NETHERLANDS WHAT’S INSIDE: WATERTOWN, CONNECTICUT, USA 2-3 – HERITAGE 4-5 – INNOVATION 6-7 – WORLD RESOURCES 8-9 – NATURAL/ORGANIC 10-11 – SILICONYL WAXES 12-13 – CUSTOM BLENDS 14-15 – EMULSIFYING WAXES 16-17 – KESTER WAXES 18-19 – MILKS 20-41 – WAX SPECIFICATIONS 42 – WAX PROPERTIES KOSTER WAX FACT: Koster Keunen was founded in the Netherlands and is world renowned for supplying quality waxes. 1852 OUR HISTORY OF TRADITION AND INNOVATION Founded in 1852 as a family business, Koster Keunen has evolved into the world’s leading processor, refiner and marketer of natural waxes. From the early days of sun bleaching beeswax for the candle industry, we now specialize in processing and formulating quality waxes for cosmetics, pharmaceutical, food, coatings, and various other technical industries worldwide. For over 150 years we have sought perfection, constantly introducing new and innovative processes and waxes, while investing in experienced, knowledgeable people and the best equipment to help meet this goal. As a family business we believe very strongly in the need for developing 3 superior quality products, and supporting our customers with excellent service, throughout the formulation and marketing processes. From our two facilities, in the USA and Holland, we offer a huge range of natural waxes, synthetic waxes and wax derivatives, enabling our customers to produce thousands of products that look, feel and work superbly KOSTERKEUNEN.COM / 1 860.945.3333 KOSTER WAX FACT: Koster Keunen was the first natural wax company to manufacture waxes using a Sandvik Pastillator, starting in 1988. 1852 UNIQUELY KOSTER KEUNEN Our greatest strength is the experience and scientific expertise we have fostered for the development of new and innovative products. -

E Number from Wikipedia, the Free Encyclopedia
E number From Wikipedia, the free encyclopedia E numbers are codes for substances which can be used as food additives for use within the European Union[1] and Switzerland (the "E" stands for "Europe").[2] They are commonly found on food labels throughout the European Union.[3] Safety assessment and approval are the responsibility of the European Food Safety Authority.[4] Having a single unified list for food additives was first agreed upon in 1962 with colours. In 1964, the directives for preservatives were added, 1970 for antioxidants and 1974 for the emulsifiers, stabilisers, thickeners and gelling agents.[5] Contents A solution of E101 riboflavin (also 1 Numbering scheme known as Vitamin B2) 2 Colloquial use 3 Classification by numeric range 4 Full list 4.1 E100–E199 (colours) 4.2 E200–E299 (preservatives) 4.3 E300–E399 (antioxidants, acidity regulators) 4.4 E400–E499 (thickeners, stabilizers, emulsifiers) 4.5 E500–E599 (acidity regulators, anti-caking Crystals of E621 Monosodium glutamate, a flavour enhancer agents) 4.6 E600–E699 (flavour enhancers) 4.7 E700–E799 (antibiotics) 4.8 E900–E999 (glazing agents and sweeteners) 4.9 E1000–E1599 (additional chemicals) 5 See also 6 Notes 7 External links Numbering scheme The numbering scheme follows that of the International Numbering System (INS) as determined by the Codex Alimentarius committee,[6] though only a subset of the INS additives are approved for use in the European Union as food additives. E numbers are also encountered on food labelling in other jurisdictions, including the Cooperation Council for the Arab States of the Gulf, Australia, New Zealand[7] and Israel. -
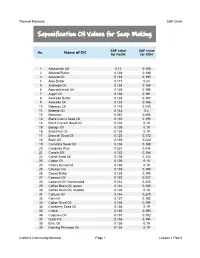
Saponification Oil Values for Soap Making
Thermal Mermaid SAP Chart Saponification Oil Values for Soap Making SAP value SAP value No. Name of Oil for NaOH for KOH 1 Abyssinian Oil 0.12 0.168 2 Almond Butter 0.134 0.188 3 Almond Oil 0.139 0.195 4 Aloe Butter 0.171 0.24 5 Andiroba Oil 0.134 0.188 6 Apricot Kernal Oil 0.139 0.195 7 Argan Oil 0.136 0.191 8 Avocado Butter 0.133 0.187 9 Avocado Oil 0.133 0.186 10 Babassu Oil 0.175 0.245 11 Baobab Oil 0.143 0.2 12 Beeswax 0.067 0.094 13 Black Cumin Seed Oil 0.139 0.195 14 Black Currant Seed Oil 0.135 0.19 15 Borage Oil 0.135 0.19 16 Brazil Nut Oil 0.135 0.19 17 Broccoli Seed Oil 0.123 0.172 18 Buriti Oil 0.159 0.223 19 Camelina Seed Oil 0.134 0.188 20 Candelila Wax 0.031 0.044 21 Canola Oil 0.133 0.186 22 Carrot Seed Oil 0.103 0.144 23 Castor Oil 0.128 0.18 24 Cherry Kernal Oil 0.135 0.19 25 Chicken Fat 0.139 0.195 26 Cocoa Butter 0.138 0.194 27 Coconut Oil 0.183 0.257 28 Coconut Oil, fractionated 0.232 0.325 29 Coffee Been Oil, green 0.132 0.185 30 Coffee Bean Oil, roasted 0.128 0.18 31 Cohune Oil 0.146 0.205 32 Corn Oil 0.137 0.192 33 Cotton Seed Oil 0.138 0.194 34 Cranberry Seed Oil 0.135 0.19 35 Crisco 0.138 0.193 36 Cupuacu Oil 0.137 0.192 37 Duck Fat 0.138 0.194 38 Emu Oil 0.135 0.19 39 Evening Primrose Oil 0.135 0.19 Crafter's Community Member Page 1 Lesson 1 Part 5 Thermal Mermaid SAP Chart SAP value SAP value No. -

Some Flow Characteristics at 37 Degrees C of Ternary Wax Mixtures
NATIONAL BUREAU OF STANDARDS REPORT 9060 Progress Report on Some Flow Characteristics at 37°C of Ternary Wax Mixtures That May Have Possible Dental Uses <&> U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. Its responsibilities include development and maintenance of the national stand- ards of measurement, and the provisions of means for making measurements consistent with those standards; determination of physical constants and properties of materials; development of methods for testing materials, mechanisms, and structures, and making such tests as may be necessary, particu- larly for government agencies; cooperation in the establishment of standard practices for incorpora- tion in codes and specifications; advisory service to government agencies on scientific and technical problems; invention and development of devices to serve special needs of the Government; assistance to industry, business, and consumers in the development and acceptance of commercial standards and simplified trade practice recommendations; administration of programs in cooperation with United States business groups and standards organizations for the development of international standards of practice; and maintenance of a clearinghouse for the collection and dissemination of scientific, tech- nical, and engineering information. The scope of the Bureau’s activities is suggested in the following listing of its four Institutes and their organizational units. Institute for Basic Standards. Applied Mathematics. Electricity. Metrology. Mechanics. Heat. Atomic Physics. Physical Chemistry. Laboratory Astrophysics.* Radiation Physics. Radio Standards Laboratory:* Radio Standards Physics; Radio Standards Engineering. -

Annex Ii Asean Guiding Principles for the Use of Additives and Excipients in Health Supplements
Association of South East Asian Nations (ASEAN) ANNEX II ASEAN GUIDING PRINCIPLES FOR THE USE OF ADDITIVES AND EXCIPIENTS IN HEALTH SUPPLEMENTS Disclaimer: This document is provided for information purpose only and subject to changes, pending the finalisation of the ASEAN Agreement on Regulatory Framework for Health Supplements. Official references to this document can only be made once the said Agreement has been finalised. Version 3.0 ASEAN Guiding Principles for the Use of Additives and Excipients in Health Supplements DOCUMENT INFORMATION This version was adopted at the 15th ASEAN HEALTH SUPPLEMENTS SCIENTIFIC COMMITTEE MEETING (ATSC) 25-27 June 2012, Singapore and endorsed at the 17th ACCSQ TRADITIONAL MEDICINES AND HEALTH SUPPLEMENTS PRODUCT WORKING GROUP (TMHSPWG) MEETING 29-30 June 2012, Singapore. History of adoption and endorsement Version Reasons for revision No. ATSC adoption date TMHSPWG endorsement date 9th ATSC Meeting 14th ACCSQ TMHS PWG 1 - 22 -23 Nov 2010 Meeting 24-25 Nov 2010 15th ATSC Meeting 17th ACCSQ TMHS PWG Amend the use of the term “national control authority” to 25-27 Jun 2012 Meeting 29-30 Jun 2012 “national control/regulatory authority”. 2 19th ATSC Meeting 19th ACCSQ TMHS PWG Update the list of adopted additives and excipients 24-27 Jun 2013 Meeting 28-29 Jun 2013 27th ATSC Meeting 23rd ACCSQ TMHS PWG 3 To limit the scope of document to Health Supplements 1-3 Jun 2015 Meeting 4-5 Jun 2015 1 of 29 Version 3.0 ASEAN Guiding Principles for the Use of Additives and Excipients in Health Supplements CONTENTS Purpose ..................................................................................................................................................... 3 Guiding Principles .................................................................................................................................. -

Gourmet Gummy Candy from Germany
Gourmet Gummy Candy from Germany Fruity-Sin is a German and American Gummy Candy company based Germany Our U.S. office is in Miami, Florida. Our Gourmet Gummies are produced in Germany. Delivery is made from Germany or Miami. Why is "Fruity-Sin" the perfect source for your sweet needs? When we say "Gourmet Gummy Candy", then we mean GOURMET. We know that we have outstanding QUALITY, this is the reason for buying from us. Why is our Gummy GOURMET? We use Real Fruit Juice. Some of them up to 50% We use Natural Fruit Sugar We use Natural Colors Some basic information: Quality Do you demand the BEST quality? We have it. Do you want to satisfy your customers? Us too. Price Quality never could be cheap, neither are we. But our product is not expensive if you consider the superior quality. Rebate We do not offer rebates, but the more you buy, the cheaper we sell. Ask for our quantity discount. MOQ Each flavor has a Minimum Order Quantity of 3.000 gram (6.6 lb) Delivery The delivery time is usually 3-4 weeks from Germany. If we have enough stock in Miami we will be much faster. Private Label We can produce any flavor, size, or shape of gourmet gummy candy. Just ask us. Shelf Life More than 12 months. Store in a cool, dry, and dark location for longest shelf life. Shelf life in this case up to 24 months. P-2 Prosecco Gold-Bears Glukosesirup, Zucker, Wasser, Gelatine, Säuerungsmittel: Citronensäure, Aroma, Farbstoffe: Echtes Karmin, Lutein, Überzugsmittel: Pflanzenöl (Kokosnuss, Raps), Carnaubawachs, 24k Gold E175 Lactose Free Gluten Free No artificial -

Synthesis and Properties of a Lacquer Wax-Based Quarternary Ammonium Gemini Surfactant
Molecules 2014, 19, 3596-3606; doi:10.3390/molecules19033596 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Synthesis and Properties of a Lacquer Wax-Based Quarternary Ammonium Gemini Surfactant Hongxia Chen 1, Chengzhang Wang 1,2,*, Jianzhong Ye 1, Hao Zhou 1,2, Li Lu 3 and Zhibing Yang 3 1 Institute of Chemical Industry of Forest Products, CAF, National Engineering Lab for Biomass Chemical Utilization; Key and Open Laboratory of Forest Chemical Engineering, SFA, Jiangsu Province, Nanjing 210042, China 2 Institute of New Technology of Forestry, CAF, Beijing 100091, China 3 Hubei Academy of Forestry, Wuhan, 430075, China * Author to whom correspondence should be addressed; E-Mails: [email protected] or [email protected]; Tel./Fax: +86-025-8548-2471. Received: 4 November 2013; in revised form: 11 March 2014 / Accepted: 14 March 2014 / Published: 24 March 2014 Abstract: Lacquer wax is an important fatty resource obtained from the mesocarp of the berries of Toxicodendron vernicifluum. In order to expand the applications of lacquer wax, we hydrolyzed it after establishing the best conditions for the acid-catalyzed hydrolysis using a Box-Behnken design. Then we synthesized a quarternary ammonium gemini surfactant by a three-step reaction. The surface properties of an aqueous solution of the final product were investigated. The optimum conditions were 9% catalyst, 100 °C of reaction temperature and 14 h of reaction time, while the maximum free fatty acids (FFA)% was 99.67%. From the gas chromatography, the main fatty acids of the lacquer wax were palmitic, oleic and octadecanoic acid. The lacquer wax gemini surfactant was synthesized, and its structure was confirmed by IR and NMR.