Maxillary Sutures As an Indicator of Adult Age at Death: Reducing Error and Codifying Approaches
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
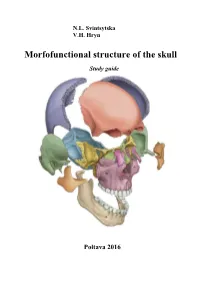
Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -
Ortho Part II
Ortho Part II Paul K. Chu, DDS St. Barnabas Hospital November 21, 2010 REVIEW FROM LAST LECTURE 1 What kinds of steps are the following? Distal Mesial Distal Mesial Moyer’s Analysis Review 1) Take an impression of a child’s MANDIBULAR arch 2) Measure the mesial distal widths of ALL permanent incisors 3) Take the number you get and look at the black row 4) The corresponding number is the mesial distal width you need for the permanent canine- 1st premolar- 2nd premolar i .e . the 3 - 4 -5 ***(Black row) ----this is the distance you measure**** 2 Moyer’s Analysis Review #1) measure the mesial distal incisal edge width of EACH permanent incisor and add them up **Let’s say in this case we measured 21mm.** Step 1 Moyer’s Analysis Review Maxilla Look at the chart Mandibular Since The resulting number measured should give you needed 21mm we look widths of the maxilla or here. mandibular space needed for permanent canines and 1st and 2nd premolars. Step 2 3 Moyer’s Analysis Review Maxilla You also use the added Mandibular measurements of the mandibular incisors to get predicted MAXILLARY measurements as well! Step 2 The Dreaded Measurements Lecture 4 What Are We Trying to Accomplish? (In other words) Is the patient Class I, II, III skeletal? Does the patient have a skeletal open bite growth pattern, or a deep bite growth pattern, or a normal growth pattern? Are the maxillary/mandibular incisors proclined, retroclined or normal? Is the facial profile protrusive, retrusive, or straight? Why? Why? Why? Why does this patient have increased -

CT of Perineural Tumor Extension: Pterygopalatine Fossa
731 CT of Perineural Tumor Extension: Pterygopalatine Fossa Hugh D. Curtin1.2 Tumors of the oral cavity and paranasal sinuses can spread along nerves to areas Richard Williams 1 apparently removed from the primary tumor. In tumors of the palate, sinuses, and face, Jonas Johnson3 this "perineural" spread usually involves the maxillary division of the trigeminal nerve. The pterygopalatine fossa is a pathway of the maxillary nerve and becomes a key landmark in the detection of neural metastasis by computed tomogaphy (CT). Oblitera tion of the fat in the fossa suggests pathology. Case material illustrating neural extension is presented and the CT findings are described. Perineural extension is possibly the most insidious form of tumor spread of head and neck malignancy. After invading a nerve, tumor follows the sheath to reach the deeper connections of the nerve, escaping the area of a planned resection. Thus, detection of this form of extension is important in treatment planning and estimation of prognosis. The pterygopalatine fossa (PPF) is a key crossroad in extension along cranial nerve V. The second branch of the trigeminal nerve passes from the gasserian ganglion through the foramen rotundum into the PPF. Here the nerve branches send communications to the palate, sinus, nasal cavity, and face. Tumor can follow any of these routes proximally into the PPF and eventually to the gasserian ganglion in the middle cranial fossa. The PPF contains enough fat to be an ideal subject for computed tomographic (CT) evaluation. Obliteration of this fat is an important indicator of pathology, including perineural tumor spread. Other signs of perineural extension include enlargement of foramina, increased enhancement in the region of Meckel cave (gasserian ganglion), and atrophy of the muscles innervated by the trigeminal nerve. -

Yagenich L.V., Kirillova I.I., Siritsa Ye.A. Latin and Main Principals Of
Yagenich L.V., Kirillova I.I., Siritsa Ye.A. Latin and main principals of anatomical, pharmaceutical and clinical terminology (Student's book) Simferopol, 2017 Contents No. Topics Page 1. UNIT I. Latin language history. Phonetics. Alphabet. Vowels and consonants classification. Diphthongs. Digraphs. Letter combinations. 4-13 Syllable shortness and longitude. Stress rules. 2. UNIT II. Grammatical noun categories, declension characteristics, noun 14-25 dictionary forms, determination of the noun stems, nominative and genitive cases and their significance in terms formation. I-st noun declension. 3. UNIT III. Adjectives and its grammatical categories. Classes of adjectives. Adjective entries in dictionaries. Adjectives of the I-st group. Gender 26-36 endings, stem-determining. 4. UNIT IV. Adjectives of the 2-nd group. Morphological characteristics of two- and multi-word anatomical terms. Syntax of two- and multi-word 37-49 anatomical terms. Nouns of the 2nd declension 5. UNIT V. General characteristic of the nouns of the 3rd declension. Parisyllabic and imparisyllabic nouns. Types of stems of the nouns of the 50-58 3rd declension and their peculiarities. 3rd declension nouns in combination with agreed and non-agreed attributes 6. UNIT VI. Peculiarities of 3rd declension nouns of masculine, feminine and neuter genders. Muscle names referring to their functions. Exceptions to the 59-71 gender rule of 3rd declension nouns for all three genders 7. UNIT VII. 1st, 2nd and 3rd declension nouns in combination with II class adjectives. Present Participle and its declension. Anatomical terms 72-81 consisting of nouns and participles 8. UNIT VIII. Nouns of the 4th and 5th declensions and their combination with 82-89 adjectives 9. -

Hard Palate, Intermaxillary Sulcus, Greater Palatine Foramen, Lesser Palatine Foramen
Basic Sciences of Medicine 2020, 9(3): 44-45 DOI: 10.5923/j.medicine.20200903.02 Twin Foramina in Posterior Third of an Adult Hard Palate and Their Significance Rajani Singh Department of Anatomy, UP University of Medical Sciences, Saifai Etawah, India Abstract Hard palate is formed by union of maxillary process of palatine bone and horizontal plate of palatine bone during development of foetus in 12th week. Three types of foramina, greater palatine allowing greater palatine nerves and vessels, lesser palatine and incisive foramina allowing passage of lesser palatine and nasopalatine nerves respectively are normally present in hard palate. The purpose of study is to report two novel foramina in hard palate and to bring out associated clinical significance. The author observed two new foramina one on either side of intermaxillary sulcus at the junction of anterior 2/3rd and posterior 1/3rd of hard palate during scanning of base of skulls for any abnormality in the Department of Anatomy of my native institute. The diameters of the right sided foramen was 6 mm while that of on left sided was 5 mm. The distance of foramen from midline on the right side was 3 mm while that of on left side was 2 mm. The distance of foramen on the right side from the centre of inferior border of hard palate was 13 mm while that of left side was 10 mm. The hard palate separates nasal cavity and oral cavity and essential for speech, feeding and respiration. The anomalous foramina observed may create problems during speech, feeding and respiration. -

Effects of Vertical Movement of the Anterior Nasal Spine on the Maxillary Stability After Lefort I Osteotomy for Pitch Correction
NAOSITE: Nagasaki University's Academic Output SITE Effects of Vertical Movement of the Anterior Nasal Spine on the Maxillary Title Stability After LeFort I Osteotomy for Pitch Correction Ohba, Seigo; Nakao, Noriko; Nakatani, Yuya; Yoshimura, Hitoshi; Author(s) Minamizato, Tokutaro; Kawasaki, Takako; Yoshida, Noriaki; Sano, Kazuo; Asahina, Izumi Citation The Journal of Craniofacial Surgery, 26(6), pp.e481-e485; 2015 Issue Date 2015-09 URL http://hdl.handle.net/10069/35883 © 2015 by Mutaz B. Habal, MD.; This is a non-final version of an article Right published in final form in The Journal of Craniofacial Surgery, 26(6), pp.e481-e485; 2015 This document is downloaded at: 2017-12-22T09:17:01Z http://naosite.lb.nagasaki-u.ac.jp Effects of vertical movement of the anterior nasal spine on the maxillary stability after LeFort I osteotomy for pitch correction Seigo Ohba, PhD 1,2, Noriko Nakao, PhD3, Yuya Nakatani1, Hitoshi Yoshimura, PhD2, Tokutaro Minamizato, PhD1, Takako Kawasaki1, Noriaki Yoshida, PhD4, Kazuo Sano, PhD2, Izumi Asahina, PhD1 1. Department of Regenerative Oral Surgery, Nagasaki University Graduate School of Biomedical Sciences 2. Division of Dentistry and Oral Surgery, Department of Sensory and Locomotor Medicine, Faculty of Medical Sciences, University of Fukui 3. Department of Special Care Dentistry, Nagasaki University Hospital of Medicine and Dentistry 4. Department of Orthodontics and Dentofacial Orthopedics, Nagasaki University Graduate School of Biomedical Sciences Corresponding author; Seigo Ohba, DDS, PhD Department of Regenerative Oral Surgery, Nagasaki University Graduate School of Biomedical Sciences Tel; +81 95 819 7704 Fax; +81 95 819 7705 e-mail; [email protected] / [email protected] Keywords; SN-PP (Palatal plane), anterior nasal spine (ANS), posterior nasal spine (PNS), clockwise rotation, counter-clockwise rotation Abstract Few reports have so far evaluated the maxillary stability after LeFort I osteotomy (L-1) for pitch correction. -

Splanchnocranium
splanchnocranium - Consists of part of skull that is derived from branchial arches - The facial bones are the bones of the anterior and lower human skull Bones Ethmoid bone Inferior nasal concha Lacrimal bone Maxilla Nasal bone Palatine bone Vomer Zygomatic bone Mandible Ethmoid bone The ethmoid is a single bone, which makes a significant contribution to the middle third of the face. It is located between the lateral wall of the nose and the medial wall of the orbit and forms parts of the nasal septum, roof and lateral wall of the nose, and a considerable part of the medial wall of the orbital cavity. In addition, the ethmoid makes a small contribution to the floor of the anterior cranial fossa. The ethmoid bone can be divided into four parts, the perpendicular plate, the cribriform plate and two ethmoidal labyrinths. Important landmarks include: • Perpendicular plate • Cribriform plate • Crista galli. • Ala. • Ethmoid labyrinths • Medial (nasal) surface. • Orbital plate. • Superior nasal concha. • Middle nasal concha. • Anterior ethmoidal air cells. • Middle ethmoidal air cells. • Posterior ethmoidal air cells. Attachments The falx cerebri (slide) attaches to the posterior border of the crista galli. lamina cribrosa 1 crista galli 2 lamina perpendicularis 3 labyrinthi ethmoidales 4 cellulae ethmoidales anteriores et posteriores 5 lamina orbitalis 6 concha nasalis media 7 processus uncinatus 8 Inferior nasal concha Each inferior nasal concha consists of a curved plate of bone attached to the lateral wall of the nasal cavity. Each consists of inferior and superior borders, medial and lateral surfaces, and anterior and posterior ends. The superior border serves to attach the bone to the lateral wall of the nose, articulating with four different bones. -

Unilateral Upper and Lower Subtotal Maxillectomy Approaches to The
NEUROSURGERY 46:6 | JUNE 2000 | 1416-1453 DOI: 10.1097/00006123-200006000-00025 Anatomic Report Unilateral Upper and Lower Subtotal Maxillectomy Approaches to the Cranial Base: Downloaded from https://academic.oup.com/neurosurgery/article-abstract/46/6/1416/2925972 by Universidad de Zaragoza user on 02 January 2020 Microsurgical Anatomy Tsutomu Hitotsumatsu, M.D., Ph.D.1, Albert L. Rhoton, Jr., M.D.1 1Department of Neurological Surgery, University of Florida, Gainesville, Florida ABSTRACT OBJECTIVE The relationship of the maxilla, with its thin walls, to the nasal and oral cavities, the orbit, and the infratemporal and pterygopalatine fossae makes it a suitable route for accessing lesions involving both the central and lateral cranial base. In this study, we compared the surgical anatomy and exposure obtained by two unilateral transmaxillary approaches, one directed through an upper subtotal maxillectomy, and the other through a lower subtotal maxillectomy. METHODS Cadaveric specimens examined, with 3 to 40× magnification, provided the material for this study. RESULTS Both upper and lower maxillectomy approaches open a surgical field extending from the ipsilateral internal carotid artery to the contralateral Eustachian tube; however, they differ in the direction of the access and the areas exposed. The lower maxillectomy opens a combination of the transmaxillary, transnasal, and transoral routes to extra- and intradural lesions of the central cranial base. Performing additional osteotomies of the mandibular coronoid process and the sphenoid pterygoid process provides anterolateral access to the lateral cranial base, including the pterygopalatine and infratemporal fossae, and the parapharyngeal space. The upper maxillectomy opens the transmaxillary and transnasal routes to the central cranial base but not the transoral route. -

Morphology of the Human Hard Palate: a Study on Dry Skulls
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Firenze University Press: E-Journals IJAE Vol. 123, n. 1: 55-63, 2018 ITALIAN JOURNAL OF ANATOMY AND EMBRYOLOGY Research Article - Basic and Applied Anatomy Morphology of the human hard palate: a study on dry skulls Masroor Badshah1,2,*, Roger Soames1, Muhammad Jaffar Khan3, Jamshaid Hasnain4 1 Centre for Anatomy and Human Identification, University of Dundee, Scotland; 2 North West School of Medicine, Hayatabad, Peshawar, Pakistan; 3 Department of Biochemistry, Khyber Medical University, Peshawar, Pakistan; 4 Bridge Consultants Foundation, Karachi, Sindh, Pakistan Abstract To determine morphological variations of the hard palate in dry human skulls, 85 skulls of unknown age and sex from nine medical schools in Khyber Pakhtunkhwa, Pakistan were exam- ined. The transverse diameter, number, shape and position of the greater (GPF) and lesser (LPF) palatine foramina; canine to canine inter-socket distance; distance between greater palatine foramen medial margins; on each side, the distances between greater palatine foramen and base of the pterygoid hamulus, median maxillary suture and posterior border of the hard palate; pal- atal length, breadth and height; maximum width and height of the incisive foramen; and the angle between the median maxillary suture and a line between the orale and greater palatine foramen were determined. Palatine index and palatal height index were also calculated. An oval greater palatine foramen was present in all skulls, while a mainly oval lesser palatine fora- men was present in 95.8% on the right and 97.2% on the left. Single and multiple lesser pala- tine foramina were observed on the right/left sides: single 44.1%/50.7%; double 41.2%/34.8%; triple 10.2%/11.6%. -

Effects of Rapid Maxillary Expansion on Upper Airway; a 3 Dimensional Cephalometric Analysis Yoon Hwan Chang Marquette University
Marquette University e-Publications@Marquette Master's Theses (2009 -) Dissertations, Theses, and Professional Projects Effects of Rapid Maxillary Expansion on Upper Airway; A 3 Dimensional Cephalometric Analysis Yoon Hwan Chang Marquette University Recommended Citation Chang, Yoon Hwan, "Effects of Rapid Maxillary Expansion on Upper Airway; A 3 Dimensional Cephalometric Analysis" (2011). Master's Theses (2009 -). Paper 85. http://epublications.marquette.edu/theses_open/85 EFFECTS OF RAPID MAXILLARY EXPANSION ON UPPER AIRWAY; A 3 DIMENSIONAL CEPHALOMETRIC ANALYSIS by Yoon H. Chang D.D.S. A Thesis submitted to the Faculty of the Graduate School, Marquette University, in Partial Fulfillment of the Requirements for the Degree of Master of Science Milwaukee, Wisconsin May 2011 ABSTRACT EFFECTS OF RAPID MAXILLARY EXPANSION ON UPPER AIRWAY; A 3 DIMENSIONAL CEPHALOMETRIC ANALYSIS Yoon H. Chang D.D.S. Marquette University, 2011 The purpose of this study was to use cone-beam computed tomography (CBCT) to assess changes in the volume and cross sectional areas of the upper airway in children with maxillary constriction treated by rapid maxillary expansion (RME). The study group consisted of 5 males and 9 females with mean age of 12.93 years with posterior cross bite and constricted maxilla who were treated with hyrax expander. Pre and post RME CBCT scans were analyzed with 3D Dolphin 11.0 software to measure the retropalatal (RP) and retroglossal (RG) airway changes. The transverse width changes were evaluated from the maxillary inter 1st molar and inter 1st pre molar mid lingual alveolar plate points. Pre and post RME scans were compared with paired t test and Pearson correlation test was done on data reaching significance. -

Posteroinferior Septal Defect Due to Vomeral Malformation
European Archives of Oto-Rhino-Laryngology (2019) 276:2229–2235 https://doi.org/10.1007/s00405-019-05443-3 RHINOLOGY Posteroinferior septal defect due to vomeral malformation Yong Won Lee1 · Young Hoon Yoon2 · Kunho Song2 · Yong Min Kim2 Received: 20 March 2019 / Accepted: 19 April 2019 / Published online: 25 April 2019 © Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract Purpose Vomeral malformation may lead to a posteroinferior septal defect (PISD). It is usually found incidentally, without any characteristic symptoms. The purpose of this study was to evaluate its clinical implications. Methods In this study, we included 18 patients with PISD after reviewing paranasal sinus computed tomography scans and medical records of 2655 patients. We evaluated the shape of the hard palate and measured the distances between the anterior nasal spine (A), the posterior end of the hard palate (P), the posterior point of the vomer fused with the palate (V), the lowest margin of the vomer at P (H), and the apex of the V-notch (N). Results None of the PISD patients had a normal posterior nasal spine (PNS). Six patients lacked a PNS or had a mild depres- sion (type 1 palate), and 12 had a V-notch (type 2 palate). The mean A–P, P–H, and P–V distances were 44.5 mm, 15.3 mm, and 12.4 mm, respectively. The average P–N distance in patients with type 2 palate was 7.3 mm. There were no statistically signifcant diferences between the types of palates in A–P, P–H, or P–V distances. -

Skull / Cranium
Important! 1. Memorizing these pages only does not guarantee the succesfull passing of the midterm test or the semifinal exam. 2. The handout has not been supervised, and I can not guarantee, that these pages are absolutely free from mistakes. If you find any, please, report to me! SKULL / CRANIUM BONES OF THE NEUROCRANIUM (7) Occipital bone (1) Sphenoid bone (1) Temporal bone (2) Frontal bone (1) Parietal bone (2) BONES OF THE VISCEROCRANIUM (15) Ethmoid bone (1) Maxilla (2) Mandible (1) Zygomatic bone (2) Nasal bone (2) Lacrimal bone (2) Inferior nasalis concha (2) Vomer (1) Palatine bone (2) Compiled by: Dr. Czigner Andrea 1 FRONTAL BONE MAIN PARTS: FRONTAL SQUAMA ORBITAL PARTS NASAL PART FRONTAL SQUAMA Parietal margin Sphenoid margin Supraorbital margin External surface Frontal tubercle Temporal surface Superciliary arch Zygomatic process Glabella Supraorbital margin Frontal notch Supraorbital foramen Internal surface Frontal crest Sulcus for superior sagittal sinus Foramen caecum ORBITAL PARTS Ethmoidal notch Cerebral surface impresiones digitatae Orbital surface Fossa for lacrimal gland Trochlear notch / fovea Anterior ethmoidal foramen Posterior ethmoidal foramen NASAL PART nasal spine nasal margin frontal sinus Compiled by: Dr. Czigner Andrea 2 SPHENOID BONE MAIN PARTS: CORPUS / BODY GREATER WINGS LESSER WINGS PTERYGOID PROCESSES CORPUS / BODY Sphenoid sinus Septum of sphenoid sinus Sphenoidal crest Sphenoidal concha Apertura sinus sphenoidalis / Opening of sphenoid sinus Sella turcica Hypophyseal fossa Dorsum sellae Posterior clinoid process Praechiasmatic sulcus Carotid sulcus GREATER WINGS Cerebral surface • Foramen rotundum • Framen ovale • Foramen spinosum Temporal surface Infratemporalis crest Infratemporal surface Orbital surface Maxillary surface LESSER WINGS Anterior clinoid process Superior orbital fissure Optic canal PTERYGOID PROCESSES Lateral plate Medial plate Pterygoid hamulus Pterygoid fossa Pterygoid sulcus Scaphoid fossa Pterygoid notch Pterygoid canal (Vidian canal) Compiled by: Dr.