33T Relation Entre Les Caractères Floraux, Le Mode De Croissance, L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Araceae) from South America and Notes on the Tribe Caladieae
Willdenowia 35 – 2005 333 JOSEF BOGNER & EDUARDO G. GONÇALVES Two new species of Xanthosoma (Araceae) from South America and notes on the tribe Caladieae Abstract Bogner, J. & Gonçalves, E. G.: Two new species of Xanthosoma (Araceae) from South America and notes on the tribe Caladieae. – Willdenowia 35: 333-344. – ISSN 0511-9618; © 2005 BGBM Berlin- Dahlem. doi:10.3372/wi.35.35216 (available via http://dx.doi.org/) Two new species of Xanthosoma sect. Acontias, X. mariae and X. latestigmatum, are described and il- lustrated. They have pilose, pedate leaf blades as have in Xanthosoma only X. plowmanii and X. pottii, and their pollen grains are released as monads, unlike in all other Xanthosoma species, which, as far as studied, release the pollen in tetrads. X. mariae is an evergreen plant mainly distinguished by its dark green velvety lustrous leaf blades with numerous leaflets and tuber-like swellings at the junction of petiole and blade; the gynoecium is of the Acontias type and the ovary is pilose in the lower part. X. latestigmatum is seasonally dormant and has medium green leaf blades with numerous leaflets and no tuber-like swellings; the gynoecium is of the Caladium type (with a very broad stigma) and completely glabrous. The relationship of the genera Caladium and Xanthosoma is discussed, C. paradoxum is transferred to Xanthosoma and the new combination X. paradoxum validated, and a key to the genera of the tribe Caladieae given. Introduction Two new species of Xanthosoma Schott cultivated in recent years in the Botanischer Garten München are described here. X. mariae has been collected only once in Peru by Mary Sizemore. -

Disentangling the Phenotypic Variation and Pollination Biology of the Cyclocephala Sexpunctata Species Complex (Coleoptera:Scara
DISENTANGLING THE PHENOTYPIC VARIATION AND POLLINATION BIOLOGY OF THE CYCLOCEPHALA SEXPUNCTATA SPECIES COMPLEX (COLEOPTERA: SCARABAEIDAE: DYNASTINAE) A Thesis by Matthew Robert Moore Bachelor of Science, University of Nebraska-Lincoln, 2009 Submitted to the Department of Biological Sciences and the faculty of the Graduate School of Wichita State University in partial fulfillment of the requirements for the degree of Master of Science July 2011 © Copyright 2011 by Matthew Robert Moore All Rights Reserved DISENTANGLING THE PHENOTYPIC VARIATION AND POLLINATION BIOLOGY OF THE CYCLOCEPHALA SEXPUNCTATA SPECIES COMPLEX (COLEOPTERA: SCARABAEIDAE: DYNASTINAE) The following faculty members have examined the final copy of this thesis for form and content, and recommend that it be accepted in partial fulfillment of the requirement for the degree of Master of Science with a major in Biological Sciences. ________________________ Mary Jameson, Committee Chair ________________________ Bin Shuai, Committee Member ________________________ Gregory Houseman, Committee Member ________________________ Peer Moore-Jansen, Committee Member iii DEDICATION To my parents and my dearest friends iv "The most beautiful thing we can experience is the mysterious. It is the source of all true art and all science. He to whom this emotion is a stranger, who can no longer pause to wonder and stand rapt in awe, is as good as dead: his eyes are closed." – Albert Einstein v ACKNOWLEDMENTS I would like to thank my academic advisor, Mary Jameson, whose years of guidance, patience and enthusiasm have so positively influenced my development as a scientist and person. I would like to thank Brett Ratcliffe and Matt Paulsen of the University of Nebraska State Museum for their generous help with this project. -

Anaphyllopsis: a New Neotropical Genus of Araceae-Lasieae
A. Hay, 1988 25 Anaphyllopsis: A New Neotropical Genus of Araceae-Lasieae Alistair Hay Department of Plant Sciences South Parks Road Oxford England During the course of revising the spe phyllous (under normal conditions of cies of Cyrtosperma in the Far East, it growth) in C. americanum, whereas in became necessary to review generic limits Dracontioides it is rhizomatous and pol in the tribe Lasieae sensu Engler ( 1911 ) as phyllous, and in Dracontium it is cormous amended by Bogner ( 1973 ). It has become and monophyllous. apparent that the pantropical Cyrtosperma It is proposed here that a new genus, circumscribed by Engler (loc. cit.) is Anaphyllopsis, be erected as an alternative heterogeneous. to "lumping" Dracontium, Dracontioides Three species of Cyrtosperma have and C. americanum. Were the latter been recognized for the New World. Two course to be adopted, the resulting broad of these belong in extant genera--C. generic concept of Dracontium would be wurdackii Bunting (Urospatba) and C. inconsistent with the existing rather nar spruceanum (Schott) Engler (Dra row limits between other genera of the contium). The necessary new combina Lasieae such as Podolasia, Urospatba, tions are to be made elsewhere, in a Lasia and Cyrtosperma s.s. forthcoming revision of Cyrtosperma. The Two new species of Anapbyllopsis are third species, C. americanum Engler, can described, both, sadly, from single frag not be fitted into any presently recognised mentary collections. Their leaf blades genus. however, are so distinctive as to justify drawing attention to these plants as repre Subtribal and generic limits in the sentatives of new and apparently rare Lasieae are also to be dealt with elsewhere. -

History and Current Status of Systematic Research with Araceae
HISTORY AND CURRENT STATUS OF SYSTEMATIC RESEARCH WITH ARACEAE Thomas B. Croat Missouri Botanical Garden P. O. Box 299 St. Louis, MO 63166 U.S.A. Note: This paper, originally published in Aroideana Vol. 21, pp. 26–145 in 1998, is periodically updated onto the IAS web page with current additions. Any mistakes, proposed changes, or new publications that deal with the systematics of Araceae should be brought to my attention. Mail to me at the address listed above, or e-mail me at [email protected]. Last revised November 2004 INTRODUCTION The history of systematic work with Araceae has been previously covered by Nicolson (1987b), and was the subject of a chapter in the Genera of Araceae by Mayo, Bogner & Boyce (1997) and in Curtis's Botanical Magazine new series (Mayo et al., 1995). In addition to covering many of the principal players in the field of aroid research, Nicolson's paper dealt with the evolution of family concepts and gave a comparison of the then current modern systems of classification. The papers by Mayo, Bogner and Boyce were more comprehensive in scope than that of Nicolson, but still did not cover in great detail many of the participants in Araceae research. In contrast, this paper will cover all systematic and floristic work that deals with Araceae, which is known to me. It will not, in general, deal with agronomic papers on Araceae such as the rich literature on taro and its cultivation, nor will it deal with smaller papers of a technical nature or those dealing with pollination biology. -

Chlorospatha Madisonii Jiff R
Prdlia, Praha, 56: i65-167, 1984 A new ardid in the Ecuadorian Andes: Clilorospatha madisonii Novy drub aronovitych v Ebadorskych Andach: Chlorospatha madisonii Jiff R. Haager and Jan J en:ik HAAGER J. R.l) et J. JENiK2) (1984): A new aroid in tlie Eeuadorian Andes: Chlaro spatha madisonii. - Preslia, Praha, 56 : 165- 167. A terrestrial aroid collected in the undergrowth of the montane rain forest, and cultivated in the greenhouse of the Pragoftora should be considered a new species of the tribe Caladieae. From the nearest Chlorospatha longipoda it differs by smal1er ha.bit, two marginal veins in the leave blade, cardinal red colouration of the sterile portion of inflorescence, black-green or browny red -green spatha, and 4- to 7-androus male flowers. 1 ) Sady, lesy a zahradnictvi Praha (Pragoflorn), Betlemskci rJ, 11000 Praha 1, Czecho slovakia. 2)' Institute of Botctny, Czechoslovak Academy of Sciences, 379 82 Tfebon, Czecho slova,kia. Spol).sored by Czech Geological Office, Pragoflora, and Czechoslovak Academy of Sciences the authors took part in a geological expedition work ing in 1981 in the environs of the Cerro Sumaco, an outlying volcano on the eastern side of the Ecuadorian Andes (HRADECKY, JAKES et al. 1983). Cerro Sumaco is the dominant summit of the Cordillera de Guarca Urcu, a north-south stretching ridge covered by montane tropical rain forests and bamboo thickets. The camping site of the expedition was situated on t,he grounds of Mr. Carlos Acosta, near Francisco de Borja, at about 1700 m altitude. Botanical observations performed by the present authors referred to the ecology and floristics of the epiphytic vegetation, and primary suc cession in t he flood-plain of Rio Quijos and Rio Borja. -

Volume 101 Annals Number 1 of the 2015 Missouri Botanical Garden
Volume 101 Annals Number 1 of the 2015 Missouri Botanical Garden A REVISION OF THE GENUS Thomas B. Croat3 and Lynn P. Hannon CHLOROSPATHA (ARACEAE)1,2 ABSTRACT This is the first revision of the genus Chlorospatha Engl. (Araceae) since Michael Madison’s 1981 treatment. The genus consists of three sections, two of which are newly established: Chlorospatha sect. Occidentales Croat & L. P. Hannon and Chlorospatha sect. Orientales Croat & L. P. Hannon. Included are 69 taxa (68 species and one variety) for Central and South America, of which 45 are new to science. These include 39 newly described species: C. bayae Croat & L. P. Hannon, C. boosii Croat & L. P. Hannon, C. bullata Croat & L. P. Hannon, C. caldasensis Croat & L. P. Hannon, C. caliensis Croat & L. P. Hannon, C. carchiensis Croat & L. P. Hannon, C. cedralensis Croat & L. P. Hannon, C. chocoensis Croat & L. P. Hannon, C. congensis Croat & L. P. Hannon, C. engleri Croat & L. P. Hannon, C. giraldoi Croat & L. P. Hannon, C. grayumii Croat & L. P. Hannon, C. hannoniae Croat, C. hastata Croat & L. P. Hannon, C. huilensis Croat & L. P. Hannon, C. jaramilloi Croat & L. P. Hannon, C. limonensis Croat & L. P. Hannon, C. litensis Croat & L. P. Hannon, C. longiloba Croat & L. P. Hannon, C. maculata Croat & L. P. Hannon, C. mansellii Croat & L. P. Hannon, C. morae Croat & L. P. Hannon, C. munchiquensis Croat & L. P. Hannon, C. nambiensis Croat & L. P. Hannon, C. narinoensis Croat & L. P. Hannon, C. noramurphyae Croat & L. P. Hannon, C. oblongifolia Croat & L. P. Hannon, C. portillae Croat & L. -

Biosystematic Studies in the Genus Anaphyllum Schott (Araceae) of Western Ghats
Int. J. LifeSc. Bt & Pharm. Res. 2012 Dominic V J, 2012 ISSN 2250-3137 www.ijlbpr.com Vol. 1, No. 3, July 2012 © 2012 IJLBPR. All Rights Reserved Research Paper BIOSYSTEMATIC STUDIES IN THE GENUS ANAPHYLLUM SCHOTT (ARACEAE) OF WESTERN GHATS Dominic V J1* *Corresponding Author: Dominic V J, [email protected] Anaphyllum Schott. is a genus belonging to the family Araceae with two species—Anaphyllum beddomei Engl. and Anaphyllum wightii Schott. These are unexplored, endemic and threatened plant species in South Western Ghats striving for their existence with treasures of genes with medicinal importance. Two variants of Anaphyllum wightii are noticed in the Western Ghats region throughout Kerala at an altitude of 650 - 1000 meters. Anaphyllum beddomei is observed only at an altitude of 1200-1500 meters from the Agusthya peaks of the South Western Ghats. Tribal communities use these plants as food and as antidote to snake bite. Two morphologically different variants of A. wightii (1-large and 2-small) were noticed in the present study. Morphological and anatomical variations were studied in A. beddomei and in the two variants of A. wightii. The Histo-taxonomical and biochemical prospecting envisaged in the present investigation reveal the important properties of this unexplored endemic and threatened genus and can lay the foundation for the complete cataloguing and indexing of the targeted species. The ISSR molecular marker studies indicate that the two variants of A. wightii with different morphologies have difference in their genetic constitution also. Keywords: Anaphyllum wightii, Genetic variation, Biosystematics, ISSR markers, CTAB method INTRODUCTION The tribal communities (Kani Tribes, Malasars, Genus Anaphyllum Schott. -

61 Floristic and Phytogeographic Aspects of Araceae in Cerro Pirre
FLORISTIC AND PHYTOGEOGRAPHIC ASPECTS OF ARACEAE IN CERRO PIRRE (DARIÉN, PANAMA) ORLANDO O. ORTIZ 1, MARÍA S. DE STAPF 1, 2, RICCARDO M. BALDINI 3 AND THOMAS B. CROAT 4 1Herbario PMA, Universidad de Panamá, Estafeta Universitaria, Ciudad de Panamá, Panamá. 2Departamento de Botánica, Universidad de Panamá, Estafeta Universitaria, Ciudad de Panamá, Panamá. 3Centro Studi Erbario Tropicale (FT herbarium) and Dipartimento di Biologia, Università di Firenze, Via La Pira 4, 50121, Firenze, Italy 4Missouri Botanical Garden, 4344 Shaw Blvd., St. Louis, MO 63110, USA. Correspondence author: Orlando O. Ortiz, [email protected] SUMMARY The aroid flora in Panama includes 436 described species in 26 genera, representing the richest country of Araceae in Central America. Much of the existing knowledge of the Panamanian aroids has been generated in the last 50 years, mainly due to extensive taxonomic studies and, to a lesser extent, by floristic studies. Floristic studies generated valuable information to better understand biodiversity, especially in the poorly- explored areas. For this reason, the main objective of this work is to study the floristic composition of the aroids of a botanically important region: Cerro Pirre (Darién Province). As a result, 430 specimens were Scientia, Vol. 28, N° 2 61 studied, comprising 94 species in 12 genera. The Aroid flora of Cerro Pirre is formed by species of wide geographic distribution (53%) and, to a lesser extent, endemic species (27%). Of the total species, approximately 43% are nomadic vines, 33% epiphytes, 23% terrestrial and a single species epilithic (1%). Ten new records for the flora of Cerro Pirre were recorded and one new record for Panama. -
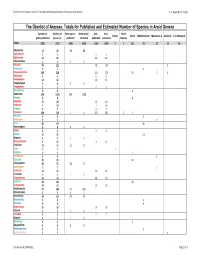
The Überlist of Araceae, Totals for Published and Estimated Number of Species in Aroid Genera P.C
The Überlist of Araceae, Totals for Published and Estimated Number of Species in Aroid Genera P.C. Boyce & T.B. Croat The Überlist of Araceae, Totals for Published and Estimated Number of Species in Aroid Genera Species per Species per Neotropical ‐ Neotropical ‐ Asia ‐ Asia ‐ North Boreal Africa Mediterranean Mascarene Is Australia Circumtropical genus published genus est. published estimated published estimated America Totals 3305 5422 1889 3143 1105 1968 2 7 152 78 20 23 29 Adelonema 13 20 13 20 Aglaodorum 11 11 Aglaonema 22 22 22 22 Alloschemone 2222 Alocasia 79 111 78 110 1 Ambrosina 11 1 Amorphophallus 196 218 153 175 35 7 1 Amydrium 55 55 Anadendrum 12 35 12 35 Anaphyllopsis 3434 Anaphyllum 22 22 Anchomanes 66 6 Anthurium 905 1500 905 1500 Anubias 88 8 Apoballis 12 20 12 20 Aridarum 716 716 Ariopsis 22 22 Arisaema 208 210 1 1 200 202 2 5 Arisarum 33 3 Arophyton 77 7 Arum 40 40 40 Asterostigma 8888 Bakoa 23 23 Biarum 21 21 21 Bognera 1111 Bucephalandra 315 315 Caladium 12 17 12 17 Calla 11 1 Callopsis 11 1 Carlephyton 33 3 Cercestis 10 10 10 Chlorospatha 28 71 28 71 Colletogyne 11 1 Colocasia 13 15 13 15 Croatiella 1111 Cryptocoryne 58 70 58 70 Culcasia 28 28 28 Cyrtosperma 13 13 13 13 Dieffenbachia 57 140 57 140 Dracontioides 2222 Dracontium 24 24 24 24 Dracunculus 22 2 Eminium 99 9 Epipremnum 15 18 15 18 Filarum 1111 Furtadoa 22 22 Gearum 1111 Gonatopus 55 5 Gorgonidium 8888 Gymnomesium 11 1 Gymnostachys 11 1 Current as of 10JAN2012 Page 1 of 3 The Überlist of Araceae, Totals for Published and Estimated Number of Species in Aroid Genera P.C. -

Leaf and Inflorescence Evidence for Near-Basal Araceae and an Unexpected Diversity of Other Monocots from the Late Early Cretaceous of Spain
Journal of Systematic Palaeontology ISSN: 1477-2019 (Print) 1478-0941 (Online) Journal homepage: http://www.tandfonline.com/loi/tjsp20 Leaf and inflorescence evidence for near-basal Araceae and an unexpected diversity of other monocots from the late Early Cretaceous of Spain Luis Miguel Sender, James A. Doyle, Garland R. Upchurch Jr, Uxue Villanueva- Amadoz & José B. Diez To cite this article: Luis Miguel Sender, James A. Doyle, Garland R. Upchurch Jr, Uxue Villanueva-Amadoz & José B. Diez (2018): Leaf and inflorescence evidence for near-basal Araceae and an unexpected diversity of other monocots from the late Early Cretaceous of Spain, Journal of Systematic Palaeontology, DOI: 10.1080/14772019.2018.1528999 To link to this article: https://doi.org/10.1080/14772019.2018.1528999 View supplementary material Published online: 09 Nov 2018. Submit your article to this journal View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tjsp20 Journal of Systematic Palaeontology, 2018 Vol. 0, No. 0, 1–34, http://doi.org/10.1080/14772019.2018.1528999 Leaf and inflorescence evidence for near-basal Araceae and an unexpected diversity of other monocots from the late Early Cretaceous of Spain aà b c d e Luis Miguel Sender , James A. Doyle , Garland R. Upchurch Jr , Uxue Villanueva-Amadoz and Jose B. Diez aDepartment of Biological Sciences, Faculty of Science and Engineering, Chuo University, 1-13-27 Kasuga, Bunkyo, Tokyo, Japan; bDepartment of Evolution and Ecology, -

Molecular Systematics and Historical Biogeography of Araceae at a Worldwide Scale and in Southeast Asia
Dissertation zur Erlangung des Doktorgrades an der Fakultät für Biologie der Ludwig-Maximilians-Universität München Molecular systematics and historical biogeography of Araceae at a worldwide scale and in Southeast Asia Lars Nauheimer München, 2. Juli 2012 Contents Table of Contents i Preface iv Statutory Declaration (Erklärung und ehrenwörtliche Versicherung) . iv List of Publications . .v Declaration of contribution as co-author . .v Notes ........................................... vi Summary . viii Zusammenfassung . ix 1 Introduction 1 General Introduction . .2 Estimating Divergence Times . .2 Fossil calibration . .2 Historical Biogeography . .3 Ancestral area reconstruction . .3 Incorporation of fossil ranges . .4 The Araceae Family . .5 General Introduction . .5 Taxonomy . .5 Biogeography . .6 The Malay Archipelago . .7 The Genus Alocasia ...................................8 Aim of this study . .9 Color plate . 10 2 Araceae 11 Global history of the ancient monocot family Araceae inferred with models accounting for past continental positions and previous ranges based on fossils 12 Supplementary Table 1: List of accessions . 25 Supplementary Table 2: List of Araceae fossils . 34 Supplementary Table 3: Dispersal matrices for ancestral area reconstruction 40 Supplementary Table 4: Results of divergence dating . 42 Supplementary Table 5: Results of ancestral area reconstructions . 45 Supplementary Figure 1: Inferred DNA substitution rates . 57 Supplementary Figure 2: Chronogram and AAR without fossil inclusion . 58 Supplementary Figure 3: Posterior distribution of fossil constraints . 59 3 Alocasia 61 Giant taro and its relatives - A phylogeny of the large genus Alocasia (Araceae) sheds light on Miocene floristic exchange in the Malesian region . 62 Supplementary Table 1: List of accessions . 71 i CONTENTS Supplementary Table 2: Crown ages of major nodes . 74 Supplementary Table 3: Clade support, divergence time estimates and ancestral area reconstruction . -

Durianology, Discovery, and Saltation — the Evolution of Aroids
Gardens’ Bulletin Singapore 71(Suppl. 2):257-313. 2019 257 doi: 10.26492/gbs71(suppl. 2).2019-20 Durianology, discovery, and saltation — the evolution of aroids A. Hay Royal Botanic Gardens Sydney, Mrs Macquarie’s Road, Sydney 2000, Australia Jardín Botánico de la Paz y Flora, Bitaco, Valle del Cauca, Colombia [email protected] “If we become attentive to natural objects, particularly living ones, in such a manner as to desire to achieve an insight into the correlation of their nature and activity, we believe ourselves best able to come to such a comprehension through a division of the parts, and this method is suitable to take us very far. With but a word one may remind the friends of science of what chemistry and anatomy have contributed to an intensive and extensive view of Nature... But these analytic efforts, continued indefinitely, produce many disadvantages. The living may indeed be separated into its elements, but one cannot put these back together and revive them. This is true even of inorganic bodies, not to mention organic ones... For this reason, the urge to cognize living forms as such, to grasp their outwardly visible and tangible parts contextually, to take them as intimations of that which is inward, and so master, to some degree, the whole in an intuition, has always arisen in men of science.” — J.W. von Goethe (1749–1832) in Brady, 2012: 272. ABSTRACT. It is argued that E.J.H. Corner’s ‘durianology’ is an integrative, holistic approach to the evolution of angiosperm form which complements reductive, atomistic phylogenetic methods involving the reification of individuated high-level abstractions in the concept of morphological ‘character evolution’.