Changes in the Vulvular and Vestibular Tissue of the Bovine During the Estrous Cycle As Determined by the Use of Near Infrared Interactance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 28 *Lecture Powepoint
Chapter 28 *Lecture PowePoint The Female Reproductive System *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • The female reproductive system is more complex than the male system because it serves more purposes – Produces and delivers gametes – Provides nutrition and safe harbor for fetal development – Gives birth – Nourishes infant • Female system is more cyclic, and the hormones are secreted in a more complex sequence than the relatively steady secretion in the male 28-2 Sexual Differentiation • The two sexes indistinguishable for first 8 to 10 weeks of development • Female reproductive tract develops from the paramesonephric ducts – Not because of the positive action of any hormone – Because of the absence of testosterone and müllerian-inhibiting factor (MIF) 28-3 Reproductive Anatomy • Expected Learning Outcomes – Describe the structure of the ovary – Trace the female reproductive tract and describe the gross anatomy and histology of each organ – Identify the ligaments that support the female reproductive organs – Describe the blood supply to the female reproductive tract – Identify the external genitalia of the female – Describe the structure of the nonlactating breast 28-4 Sexual Differentiation • Without testosterone: – Causes mesonephric ducts to degenerate – Genital tubercle becomes the glans clitoris – Urogenital folds become the labia minora – Labioscrotal folds -

Chapter 24 Primary Sex Organs = Gonads Produce Gametes Secrete Hormones That Control Reproduction Secondary Sex Organs = Accessory Structures
Anatomy Lecture Notes Chapter 24 primary sex organs = gonads produce gametes secrete hormones that control reproduction secondary sex organs = accessory structures Development and Differentiation A. gonads develop from mesoderm starting at week 5 gonadal ridges medial to kidneys germ cells migrate to gonadal ridges from yolk sac at week 7, if an XY embryo secretes SRY protein, the gonadal ridges begin developing into testes with seminiferous tubules the testes secrete androgens, which cause the mesonephric ducts to develop the testes secrete a hormone that causes the paramesonephric ducts to regress by week 8, in any fetus (XX or XY), if SRY protein has not been produced, the gondal ridges begin to develop into ovaries with ovarian follicles the lack of androgens causes the paramesonephric ducts to develop and the mesonephric ducts to regress B. accessory organs develop from embryonic duct systems mesonephric ducts / Wolffian ducts eventually become male accessory organs: epididymis, ductus deferens, ejaculatory duct paramesonephric ducts / Mullerian ducts eventually become female accessory organs: oviducts, uterus, superior vagina C. external genitalia are indeterminate until week 8 male female genital tubercle penis (glans, corpora cavernosa, clitoris (glans, corpora corpus spongiosum) cavernosa), vestibular bulb) urethral folds fuse to form penile urethra labia minora labioscrotal swellings fuse to form scrotum labia majora urogenital sinus urinary bladder, urethra, prostate, urinary bladder, urethra, seminal vesicles, bulbourethral inferior vagina, vestibular glands glands Strong/Fall 2008 Anatomy Lecture Notes Chapter 24 Male A. gonads = testes (singular = testis) located in scrotum 1. outer coverings a. tunica vaginalis =double layer of serous membrane that partially surrounds each testis; (figure 24.29) b. -

The Reproductive System
27 The Reproductive System PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • The reproductive system is designed to perpetuate the species • The male produces gametes called sperm cells • The female produces gametes called ova • The joining of a sperm cell and an ovum is fertilization • Fertilization results in the formation of a zygote © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • Overview of the Male Reproductive System • Testis • Epididymis • Ductus deferens • Ejaculatory duct • Spongy urethra (penile urethra) • Seminal gland • Prostate gland • Bulbo-urethral gland © 2012 Pearson Education, Inc. Figure 27.1 The Male Reproductive System, Part I Pubic symphysis Ureter Urinary bladder Prostatic urethra Seminal gland Membranous urethra Rectum Corpus cavernosum Prostate gland Corpus spongiosum Spongy urethra Ejaculatory duct Ductus deferens Penis Bulbo-urethral gland Epididymis Anus Testis External urethral orifice Scrotum Sigmoid colon (cut) Rectum Internal urethral orifice Rectus abdominis Prostatic urethra Urinary bladder Prostate gland Pubic symphysis Bristle within ejaculatory duct Membranous urethra Penis Spongy urethra Spongy urethra within corpus spongiosum Bulbospongiosus muscle Corpus cavernosum Ductus deferens Epididymis Scrotum Testis © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • The Testes • Testes hang inside a pouch called the scrotum, which is on the outside of the body -

New Insights Into Human Female Reproductive Tract Development
UCSF UC San Francisco Previously Published Works Title New insights into human female reproductive tract development. Permalink https://escholarship.org/uc/item/7pm5800b Journal Differentiation; research in biological diversity, 97 ISSN 0301-4681 Authors Robboy, Stanley J Kurita, Takeshi Baskin, Laurence et al. Publication Date 2017-09-01 DOI 10.1016/j.diff.2017.08.002 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Differentiation 97 (2017) xxx–xxx Contents lists available at ScienceDirect Differentiation journal homepage: www.elsevier.com/locate/diff New insights into human female reproductive tract development MARK ⁎ Stanley J. Robboya, , Takeshi Kuritab, Laurence Baskinc, Gerald R. Cunhac a Department of Pathology, Duke University, Davison Building, Box 3712, Durham, NC 27710, United States b Department of Cancer Biology and Genetics, The Comprehensive Cancer Center, Ohio State University, 460 W. 12th Avenue, 812 Biomedical Research Tower, Columbus, OH 43210, United States c Department of Urology, University of California, 400 Parnassus Avenue, San Francisco, CA 94143, United States ARTICLE INFO ABSTRACT Keywords: We present a detailed review of the embryonic and fetal development of the human female reproductive tract Human Müllerian duct utilizing specimens from the 5th through the 22nd gestational week. Hematoxylin and eosin (H & E) as well as Urogenital sinus immunohistochemical stains were used to study the development of the human uterine tube, endometrium, Uterovaginal canal myometrium, uterine cervix and vagina. Our study revisits and updates the classical reports of Koff (1933) and Uterus Bulmer (1957) and presents new data on development of human vaginal epithelium. Koff proposed that the Cervix upper 4/5ths of the vagina is derived from Müllerian epithelium and the lower 1/5th derived from urogenital Vagina sinus epithelium, while Bulmer proposed that vaginal epithelium derives solely from urogenital sinus epithelium. -

4 Lecture Uterus Gross Anatomy
Body: major portion Uterine body Fundus: rounded superior region Fundus Isthmus: narrowed inferior region Lumen of uterus Cervix: narrow neck (cavity) of uterus Wall of uterus Body of uterus • Endometrium • Myometrium • Perimetrium Isthmus Cervical canal Vagina Cervix Posterior view © 2016 Pearson Education, Inc. Uterus: ligaments (woman) The ligaments of the uterus are 10 in number: one anterior (vesicouterine fold of peritoneum); one posterior (rectouterine fold of peritoneum); two lateral or broad; two uterosacral; two cardinal (lateral cervical) ligaments; and two round ligaments. Anterior ligament: consists of the vesicouterine fold of peritoneum, which is reflected on to the bladder from the front of the uterus Posterior ligament: consists of the rectouterine fold of peritoneum, which is reflected from cervix on to the front of the rectum. Uterosacral ligaments: secure uterus to sacrum Suspensory ligament of ovary Peritoneum Uterine tube Ovary Uterosacral ligament Uterus Rectouterine Round ligament pouch Vesicouterine pouch Rectum Urinary bladder Pubic symphysis Mons pubis Cervix Urethra Clitoris Vagina External urethral orifice Anus © 2016 Pearson Education, Inc. Domestic animals Rectum rectouterine fold vesicouterine fold Bladder cardinal (lateral cervical) ligaments: from cervix and superior vagina to pelvic lateral walls Suspensory ligament of Uterine ovary (fallopian) tube Fundus Lumen of uterus Ovarian (cavity) blood vessels of uterus Uterine tube Broad ligament Ovary • Ampulla • Isthmus • Mesosalpinx • Infundibulum • Mesovarium -

Colposcopy of the Uterine Cervix
THE CERVIX: Colposcopy of the Uterine Cervix • I. Introduction • V. Invasive Cancer of the Cervix • II. Anatomy of the Uterine Cervix • VI. Colposcopy • III. Histology of the Normal Cervix • VII: Cervical Cancer Screening and Colposcopy During Pregnancy • IV. Premalignant Lesions of the Cervix The material that follows was developed by the 2002-04 ASCCP Section on the Cervix for use by physicians and healthcare providers. Special thanks to Section members: Edward J. Mayeaux, Jr, MD, Co-Chair Claudia Werner, MD, Co-Chair Raheela Ashfaq, MD Deborah Bartholomew, MD Lisa Flowers, MD Francisco Garcia, MD, MPH Luis Padilla, MD Diane Solomon, MD Dennis O'Connor, MD Please use this material freely. This material is an educational resource and as such does not define a standard of care, nor is intended to dictate an exclusive course of treatment or procedure to be followed. It presents methods and techniques of clinical practice that are acceptable and used by recognized authorities, for consideration by licensed physicians and healthcare providers to incorporate into their practice. Variations of practice, taking into account the needs of the individual patient, resources, and limitation unique to the institution or type of practice, may be appropriate. I. AN INTRODUCTION TO THE NORMAL CERVIX, NEOPLASIA, AND COLPOSCOPY The uterine cervix presents a unique opportunity to clinicians in that it is physically and visually accessible for evaluation. It demonstrates a well-described spectrum of histological and colposcopic findings from health to premalignancy to invasive cancer. Since nearly all cervical neoplasia occurs in the presence of human papillomavirus infection, the cervix provides the best-defined model of virus-mediated carcinogenesis in humans to date. -

January 2019 ASC Fee Schedule
Montana Healthcare Programs Fee Schedule ASC Covered Surgical and Ancillary Services January 1, 2019 Proc Code Proc Description Multiple Procedure PA Effective Method Fee 0042T CT PERFUSION W/CONTRAST CBF - - 7/1/2018 Not Allowed $0.00 0054T BONE SRGRY CMPTR FLUOR IMAGE - - 7/1/2018 Not Allowed $0.00 0055T BONE SRGRY CMPTR CT/MRI IMAG - - 7/1/2018 Not Allowed $0.00 0071T US LEIOMYOMATA ABLATE <200 - Y 7/1/2018 Not Allowed $0.00 0072T US LEIOMYOMATA ABLATE >200 - Y 7/1/2018 Not Allowed $0.00 0100T PROSTH RETINA RECEIVE&GEN - Y 7/1/2018 Not Allowed $0.00 0101T EXTRACORP SHOCKWV TX HI ENRG - Y 7/1/2018 Not Allowed $0.00 0102T EXTRACORP SHOCKWV TX ANESTH - Y 7/1/2018 Not Allowed $0.00 0126T CHD RISK IMT STUDY - Y 7/1/2018 Not Allowed $0.00 0159T CAD BREAST MRI - - 7/1/2018 Not Allowed $0.00 0174T CAD CXR WITH INTERP - - 7/1/2018 Not Allowed $0.00 0175T CAD CXR REMOTE - - 7/1/2018 Not Allowed $0.00 0191T INSERT ANT SEGMENT DRAIN INT - Y 7/1/2018 Not Allowed $0.00 0200T PERQ SACRAL AUGMT UNILAT INJ - Y 7/1/2018 Not Allowed $0.00 0201T PERQ SACRAL AUGMT BILAT INJ - Y 7/1/2018 Not Allowed $0.00 0205T INIRS EACH VESSEL ADD-ON - - 7/1/2018 Not Allowed $0.00 0206T CPTR DBS ALYS CAR ELEC DTA - - 7/1/2018 Not Allowed $0.00 0213T NJX PARAVERT W/US CER/THOR - Y 7/1/2018 Not Allowed $0.00 0214T NJX PARAVERT W/US CER/THOR - Y 7/1/2018 No Separate Payment $0.00 0215T NJX PARAVERT W/US CER/THOR - Y 7/1/2018 No Separate Payment $0.00 0216T NJX PARAVERT W/US LUMB/SAC - Y 7/1/2018 Not Allowed $0.00 0217T NJX PARAVERT W/US LUMB/SAC - Y 7/1/2018 No Separate -
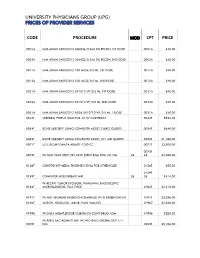
Code Procedure Cpt Price University Physicians Group
UNIVERSITY PHYSICIANS GROUP (UPG) PRICES OF PROVIDER SERVICES CODE PROCEDURE MOD CPT PRICE 0001A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 1ST DOSE 0001A $40.00 0002A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 2ND DOSE 0002A $40.00 0011A IMM ADMN SARSCOV2 100 MCG/0.5 ML 1ST DOSE 0011A $40.00 0012A IMM ADMN SARSCOV2 100 MCG/0.5 ML 2ND DOSE 0012A $40.00 0021A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 1ST DOSE 0021A $40.00 0022A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 2ND DOSE 0022A $40.00 0031A IMM ADMN SARSCOV2 AD26 5X10^10 VP/0.5 ML 1 DOSE 0031A $40.00 0042T CEREBRAL PERFUS ANALYSIS, CT W/CONTRAST 0042T $954.00 0054T BONE SURGERY USING COMPUTER ASSIST, FLURO GUIDED 0054T $640.00 0055T BONE SURGERY USING COMPUTER ASSIST, CT/ MRI GUIDED 0055T $1,188.00 0071T U/S LEIOMYOMATA ABLATE <200 CC 0071T $2,500.00 0075T 0075T PR TCAT PLMT XTRC VRT CRTD STENT RS&I PRQ 1ST VSL 26 26 $2,208.00 0126T CAROTID INT-MEDIA THICKNESS EVAL FOR ATHERSCLER 0126T $55.00 0159T 0159T COMPUTER AIDED BREAST MRI 26 26 $314.00 PR RECTAL TUMOR EXCISION, TRANSANAL ENDOSCOPIC 0184T MICROSURGICAL, FULL THICK 0184T $2,315.00 0191T PR ANT SEGMENT INSERTION DRAINAGE W/O RESERVOIR INT 0191T $2,396.00 01967 ANESTH, NEURAXIAL LABOR, PLAN VAG DEL 01967 $2,500.00 01996 PR DAILY MGMT,EPIDUR/SUBARACH CONT DRUG ADM 01996 $285.00 PR PERQ SAC AGMNTJ UNI W/WO BALO/MCHNL DEV 1/> 0200T NDL 0200T $5,106.00 PR PERQ SAC AGMNTJ BI W/WO BALO/MCHNL DEV 2/> 0201T NDLS 0201T $9,446.00 PR INJECT PLATELET RICH PLASMA W/IMG 0232T HARVEST/PREPARATOIN 0232T $1,509.00 0234T PR TRANSLUMINAL PERIPHERAL ATHERECTOMY, RENAL -

Female Genital System
The University Of Jordan Faculty Of Medicine Female genital system By Dr.Ahmed Salman Assistant Professor of Anatomy &Embryology Female Genital Organs This includes : 1. Ovaries 2. Fallopian tubes 3. Uterus 4. Vagina 5. External genital organs Ovaries Site of the Ovary: In the ovarian fossa in the lateral wall of the pelvis which is bounded. Anteriorly : External iliac vessels. Posteriorly : internal iliac vessels and ureter. Shape : the ovary is almond-shaped. Orientation : In the nullipara : long axis is vertical with superior and inferior poles. In multipara : long axis is horizontal, so that the superior pole is directed laterally and the inferior pole is directed medially. External Features : Before puberty : Greyish-pink and smooth. After puberty with onset of ovulation, the ovary becomes grey in colour with puckered surface. In old age : it becomes atrophic External iliac vessels. Obturator N. Internal iliac artery Ureter UTERUS Ovaries Description : In nullipara, the ovary has : Two ends : superior (tubal) end and inferior (uterine) end. Two borders : anterior (mesovarian) border and posterior (free) border. Two surfaces : lateral and medial. A. Ends of the Ovary : Superior (tubal) end : is attached to the ovarian fimbria of the uterine tube and is attached to side wall of the pelvis by the ovarian suspensory ligament. Inferior (uterine) end : it is connected to superior aspect of the uterotubal junction by the round ligament of the ovary which runs within the broad ligament . B. Borders of the Ovary : Anterior (mesovarian) border :presents the hilum of the ovary and is attached to the upper layer of the broad ligament by a short peritoneal fold called the mesovarium. -

The Physiology of the Uterus in Labor
The Physiology of the Uterus in Labor D. N. DANFORTH, M.D., R. J. GRAHAM, B.M. and A. C. IVY, M.D.l HE purpose of this article is to uterine horns (8). This ring becomes T synthesize into a rather complete fairly evident during labor at the junc picture the experimental observations tion of the upper and lower segments. that have been made during the past It is a more or less definite, tapering ten years in our laboratory regarding ledge, the formation of which is due the processes concerned in the evacu to the greater thickness or "retraction" ation of the uterus. of the muscle fibers of the upper than the lower segment. (This r ing is the The Anatomic and Physiologic " fundal or cornal sphincter" in the Divisions of the Uterus dog.) In obstructed labors it becomes It is important first to obtain a clear a very pronounced ring or band and concept (a) of the anatomical divisions is then called Bandl's ring. The ap of the human uterus, which may be pearance of a Bandl's ring means -- traced directly to those in lower forms, threatened rupture of the uterus in the and (b) of the obstetrical or physio lower segment ; it is a pathological re logical divisions. See figure 1. traction ring. This is a subject that has been greatly III. The lower uterine segment is an confused because our knowledge of the alogous to the isthmus uteri oj Asch gross and microscopic anatomy of the off in the non-pregnant uterus. In human uterus, and of the embryology, woman its upper level is generally comparative anatomy, and physiology marked by the reflection of the per of the uterus has developed in ~ hap itoneum. -

Repo. Bookwork
Male and Female Reproductive System Bookwork ***YOU DO NOT NEED TO KNOW THE BLUE QUESTIONS- they will NOT be on the test! #2. Which of these is mismatched? a.) Vagina-Penis b.) Testis-Ovaries c.) Labia Majora-scrotum d.) Oviduct-Ductus Deferens ! #3. The myometrium is the muscular layer of the uterus, and the endometrium is the ___________ layer. a.) Serosa b.) adventitia c.) Submucosa d.) Mucosa ! #5. The approximate area between the anus and the clitoris in the female is the: a.) Peritoneum b.) perineum c.) vulva d.) Labia ! #6. Which of the following attach to the ovary? a.) Fimbriae b.) mesosalpinx c.) suspensory ligaments d.) broad ligament ! #7. Human ova and sperm are similar in that: a.) About the same number of each is produces each month b.) They have the same degree of motility c.) They are about the same size d.) They have the same number of chromosomes ! #8. Select the FALSE statement about the cervix of the uterus: a.) It is the superior most part of the uterus. b.) It projects into the vagina c.) Its cervical glands secrete mucus d.) It contains the cervical canal ! #9. Each month, only one: a.) Primary follicle is stimulated b.) Follicle secretes estrogen c.) Vesicular follicle undergoes ovulation d.) Ovary is stimulated ! #10. After ovulation, the ruptured follicle: a.) degenerates b.) becomes a corpus luteum c.) sloughs off as waste material d.) Mends and produces another oocyte. ! #11. The outer layer of the blastocyst, which attaches to the uterine wall, is the : a.) Yolk sac b.) inner cell mass c.) Amnion d.) trophoblast ! #12. -

Alekls0201b.Pdf
Female genital system Miloš Grim Institute of Anatomy, First Faculty of Medicine, Summer semester 2017 / 2018 Female genital system Internal genital organs Ovary, Uterine tube- Salpinx, Fallopian tube, Uterus - Metra, Hystera, Vagina, colpos External genital organs Pudendum- vulva, cunnus Mons pubis Labium majus Pudendal cleft Labium minus Vestibule Bulb of vestibule Clitoris MRI of female pelvis in sagittal plane Female pelvis in sagittal plane Internal genital organs of female genital system Ovary, Uterine tube, Uterus, Broad ligament of uterus, Round lig. of uterus Anteflexion, anteversion of uterus Transverse section through the lumbar region of a 6-week embryo, colonization of primitive gonade by primordial germ cells Primordial germ cells migrate into gonads from the yolk sac Differentiation of indifferent gonads into ovary and testis Ovary: ovarian follicles Testis: seminiferous tubules, tunica albuginea Development of broad ligament of uterus from urogenital ridge Development of uterine tube, uterus and part of vagina from paramesonephric (Mullerian) duct Development of position of female internal genital organs, ureter Broad ligament of uterus Transverse section of female pelvis Parametrium Supporting apparatus of uterus, cardinal lig. (broad ligament) round ligament pubocervical lig. recto-uterine lig. Descent of ovary. Development of uterine tube , uterus and part of vagina from paramesonephric (Mullerian) duct External genital organs develop from: genital eminence, genital folds, genital ridges and urogenital sinus ureter Broad ligament of uterus Transverse section of female pelvis Ovary (posterior view) Tubal + uterine extremity, Medial + lateral surface Free + mesovarian border, Mesovarium, Uteroovaric lig., Suspensory lig. of ovary, Mesosalpinx, Mesometrium Ovary, uterine tube, fimbrie of the tube, fundus of uterus Ovaric fossa between internal nd external iliac artery Sagittal section of plica lata uteri (broad lig.