Benthic Metabolism in Two Turbid Dryland Rivers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lower Cretaceous Fossils from the Sources of the Barcoo, Ward And
LOWER CRETACEOUS FOSSILS FROM THE SOURCE8 OF THE BARCOO, WARD AND NIV E RIVERS SOUTH OENTRAL QUEENSLA~D. PART II.l-CEPHALOPODA. By R. ETHERIDGE, JUNR., Curator. (Plates xxx.-xlix., and Fig. 8.) I.-IN'l'RODUC'l'ION. The" Ammonites" proper contained in Mr. H. \V. Blomfield's collection are few in nnmber', and will be noticed in a later part of this Memoir. The following descriptions of the "Crioceri" are offered with a considerable amsmnt of diffidence, and to arrive at these results I found it impemtive to undertake a general review of our Cretaceous forms hitherto associated with the names CTioceras and Ancylocera8; the outcome of this work, extending over many months, is t~ my mind, I regret to say, anything but satisfactory. This arises from two primary causes, the frag mentary state in which the fossils are frequently found, and the difficulty experienced in grouping ~uch portions, from many and "widely separated localities, in well dlefined species. A secondary cause is due to the fact that many of the hitherto described forms are based on portions of shells only, and in two instances at least the descriptions are so inadequate that recognition of the species is practically reduced to gue~s work. In no instance is this more apparent than in that of Cl·iocemB australe, Moore, the Australian type of the group. To render my investigations as complete as possible, I assembled all the specimens of the above two genera within my reach, and drew upon the following collections, as well as onr own :-The Mining and Geological, and Macleay Museums, ~ydney; National Museum, Melbourne; Geological Survey, and Queensland Museums, Brisbane; Geological Survey Collection, Adelaide; and the Sweet Collection, Melbourne. -

Restricted Water Ski Areas in Queensland
Restricted Water Ski areas in Queensland Watercourse Date of Gazettal Any person operating a ship towing anyone by a line attached to the ship (including for example a person water skiing or riding on a toboggan or tube) within the waters listed below endangers marine safety. Brisbane River 20/10/2006 South Brisbane and Town Reaches of the Brisbane River between the Merivale Bridge and the Story Bridge. Burdekin River, Charters Towers 13/09/2019 All waters of The Weir on the Burdekin River, Charters Towers. Except: • commencing at a point on the waterline of the eastern bank of the Burdekin River nearest to location 19°55.279’S, 146°16.639’E, • then generally southerly along the waterline of the eastern bank to a point nearest to location 19°56.530’S, 146°17.276’E, • then westerly across Burdekin River to a point on the waterline of the western bank nearest to location 19°56.600’S, 146°17.164’E, • then generally northerly along the waterline of the western bank to a point on the waterline nearest to location 19°55.280’S, 146°16.525’E, • then easterly across the Burdekin River to the point of commencement. As shown on the map S8sp-73 prepared by Maritime Safety Queensland (MSQ) which can be found on the MSQ website at www.msq.qld.gov.au/s8sp73map and is held at MSQ’s Townsville Office. Burrum River .12/07/1996 The waters of the Burrum River within 200 metres north from the High Water mark of the southern river bank and commencing at a point 50 metres downstream of the public boat ramp off Burrum Heads Road to a point 200 metres upstream of the upstream boundary of Lions Park, Burrum Heads. -
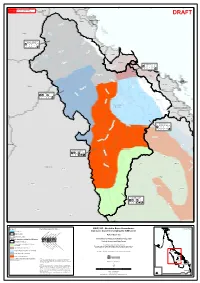
GWQ1203 - Burdekin Basin Groundwater Location Map Watercourse Cainozoic Deposits Overlying the GAB Zones
144°0'0"E 145°0'0"E 146°0'0"E 147°0'0"E 148°0'0"E 149°0'0"E Tully 18°0'0"S This map must not be used for marine navigation. 18°0'0"S Comprehensive and updated navigation WARNING information should be obtained from published Mitchell hydrographic charts. Murray DRAFT Hinchinbrook Island Herbert r ve Ri ekin urd B Ingham ! er iv D R ry Gilbert ry Ri D v er r e iv 19°0'0"S Scattered Remnants Northern R 19°0'0"S g Burdekin Headwaters in n n R u Black Townsville ! er r iv e R v rke i la R C r ta S Ross Broken River Ayr ! er ver Riv i ry lt R Haughton go Basa e r Don and Bogie Coastal Area G h t r u ve o i ton R S h ug Ha Bowen ! 20°0'0"S 20°0'0"S r Charters Towers e v ! i Don R B n o o g i D e Riv er Bur dek in R ive r Whitsunday Island Proserpine B owe Proserpine Flinders n ! er R Riv iver e sp a R amp C o Don River l l s to n R iv C e a r North West Suttor Catchment pe R r iv ve e i r R r o t t u S r e Broken v R i iv S e R e r llh L e e i O'Connell p im t t a l R e C iv er B o w e B u r d ekin n B u r d ekin R 21°0'0"S i 21°0'0"S v e BB a a s s in in r B ro Mackay ke ! n River Pioneer Plane Sarina ! Eastern Weathered Su ttor R Cainozoic Remnants Belyandor River iv e er iv R o d n lya e B r e v i R o d Moranbah n ! a ly 22°0'0"S e 22°0'0"S l R B ae iver ich Carm r ive R Diamantina s r o n n o C Styx Belyando River Saline Tertiary Sediments Dysart ! Is a a c R iv B Clermont Middlemount er e ! ! l y an do Riv e r er iv Cooper Creek R ie nz Aramac e ! k c a M 23°0'0"S 23°0'0"S Capella -

Innamincka/Cooper Creek State Heritage Area Innamincka/Cooper Creek Was Declared a State Heritage Area on 16 May 1985
Innamincka/Cooper Creek State Heritage Area Innamincka/Cooper Creek was declared a State Heritage Area on 16 May 1985. HISTORY Innamincka and Cooper Creek have a rich history. The area has also been associated with many other facets of the state's history since colonial settlement. Names such as Charles Sturt, Sidney Kidman, Cordillo Downs, the Australian Inland Mission and the Reverend John Flynn are intrinsically woven into the stories of Innamincka and the Cooper Creek. Its significance for Aboriginal people spans thousands of years as a trade route and a source of abundant food and fresh water. The area's Aboriginal history also includes significant contacts with explorers, pastoralists and settlers. Many sources believe that the name 'Innamincka' comes from two Aboriginal words meaning ' your shelter' or 'your home'. European contact with the region came first with the explorers and later with the establishment of the pastoral industry, transport routes and service centres. Cooper Creek was named by Captain Charles Sturt on 13 October 1845, when he crossed the watercourse at a point approximately 24 kilometres west of the current Innamincka township. The water was very low at the time, hence his use of the term 'creek' rather than 'river' to describe what often becomes a deep torrent of water. Charles Cooper, after whom the waterway was named, was a South Australian judge - later Chief Justice Sir Charles Cooper. On the same expedition, Sturt also named the Strzelecki Desert after the eccentric Polish explorer, Paul Edmund de Strzelecki, who was the first European to climb and name Mount Kosciusko. -

Surface Water Ambient Network (Water Quality) 2020-21
Surface Water Ambient Network (Water Quality) 2020-21 July 2020 This publication has been compiled by Natural Resources Divisional Support, Department of Natural Resources, Mines and Energy. © State of Queensland, 2020 The Queensland Government supports and encourages the dissemination and exchange of its information. The copyright in this publication is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) licence. Under this licence you are free, without having to seek our permission, to use this publication in accordance with the licence terms. You must keep intact the copyright notice and attribute the State of Queensland as the source of the publication. Note: Some content in this publication may have different licence terms as indicated. For more information on this licence, visit https://creativecommons.org/licenses/by/4.0/. The information contained herein is subject to change without notice. The Queensland Government shall not be liable for technical or other errors or omissions contained herein. The reader/user accepts all risks and responsibility for losses, damages, costs and other consequences resulting directly or indirectly from using this information. Summary This document lists the stream gauging stations which make up the Department of Natural Resources, Mines and Energy (DNRME) surface water quality monitoring network. Data collected under this network are published on DNRME’s Water Monitoring Information Data Portal. The water quality data collected includes both logged time-series and manual water samples taken for later laboratory analysis. Other data types are also collected at stream gauging stations, including rainfall and stream height. Further information is available on the Water Monitoring Information Data Portal under each station listing. -

100 the SOUTH-WEST CORNER of QUEENSLAND. (By S
100 THE SOUTH-WEST CORNER OF QUEENSLAND. (By S. E. PEARSON). (Read at a meeting of the Historical Society of Queensland, August 27, 1937). On a clear day, looking westward across the channels of the Mulligan River from the gravelly tableland behind Annandale Homestead, in south western Queensland, one may discern a long low line of drift-top sandhills. Round more than half the skyline the rim of earth may be likened to the ocean. There is no break in any part of the horizon; not a landmark, not a tree. Should anyone chance to stand on those gravelly rises when the sun was peeping above the eastem skyline they would witness a scene that would carry the mind at once to the far-flung horizons of the Sahara. In the sunrise that western region is overhung by rose-tinted haze, and in the valleys lie the purple shadows that are peculiar to the waste places of the earth. Those naked, drift- top sanddunes beyond the Mulligan mark the limit of human occupation. Washed crimson by the rising sun they are set Kke gleaming fangs in the desert's jaws. The Explorers. The first white men to penetrate that line of sand- dunes, in south-western Queensland, were Captain Charles Sturt and his party, in September, 1845. They had crossed the stony country that lies between the Cooper and the Diamantina—afterwards known as Sturt's Stony Desert; and afterwards, by the way, occupied in 1880, as fair cattle-grazing country, by the Broad brothers of Sydney (Andrew and James) under the run name of Goyder's Lagoon—and the ex plorers actually crossed the latter watercourse with out knowing it to be a river, for in that vicinity Sturt describes it as "a great earthy plain." For forty miles one meets with black, sundried soil and dismal wilted polygonum bushes in a dry season, and forty miles of hock-deep mud, water, and flowering swamp-plants in a wet one. -

FLOOD WARNING SYSTEM for the COOPER CREEK CATCHMENT
Bureau Home > Australia > Queensland > Rainfall & River Conditions > River Brochures > Thomson, Barcoo and Cooper FLOOD WARNING SYSTEM for the COOPER CREEK CATCHMENT This brochure describes the flood warning system operated by the Australian Government, Bureau of Meteorology for the Thomson, Barcoo Rivers and Cooper Creek. It includes reference information which will be useful for understanding Flood Warnings and River Height Bulletins issued by the Bureau's Flood Warning Centre during periods of high rainfall and flooding. Contained in this document is information about: (Last updated September 2019) Flood Risk Previous Flooding Flood Forecasting Local Information Flood Warnings and Bulletins Interpreting Flood Warnings and River Height Bulletins Flood Classifications Other Links Cooper Creek at Nappa Merrie Flood Risk The Thomson-Barcoo-Cooper catchment drains an area of approximately 237,000 square kilometres and is the largest river basin in Queensland. The catchment falls within the Lake Eyre basin, the largest and only co- ordinated internal drainage system in Australia with no external outlet, and which covers over 1.1 million square kilometres of central Australia. Floodwaters reach Lake Eyre after major flood events in the Cooper. The two main tributaries, the Thomson and Barcoo Rivers, merge into the Cooper Creek approximately 40 kilometres upstream of Windorah. The Thomson River and its tributaries flow in a general southerly direction and has several of the larger towns of the region including Longreach and Muttaburra along its banks. The Barcoo River flows in a general westerly direction and has major centres such as Isisford, Blackall, Barcaldine, Jericho and Tambo in its catchment. The Thomson-Barcoo-Cooper basin can be divided into two distinct areas: * Above Windorah, numerous well-defined creeks and channels flow into the Thomson and Barcoo. -
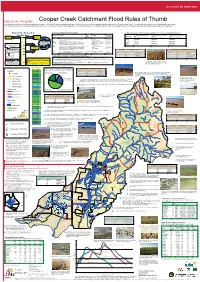
Cooper Creek Catchment Flood Rules of Thumb This Guide Has Used the Best Information Available at Present
QueenslandQueenslandthethe Smart Smart State State How to use this guide: Cooper Creek Catchment Flood Rules of Thumb This guide has used the best information available at present. It is intended to help you assess what type of flood is likely to occur in your area and indicate what amount of feed you might expect. You may wish to record your own flooding guides on the map. You can add more value to this guide by participating in an MLA EDGEnetwork Grazing Land Management (GLM) training package. GLM training helps you identify land types and flood zones and to develop a grazing management plan for your property Amount of rain needed Channel Country Flood descriptions Estimated Summer Flood Pasture Growth in the Channel Country Floodplains. Frequently flooded plains Occasionally flooded plains Swamps and depressions for flooding Flood type Description Land Hydrology Pasture growth Isolated Systems which supports: Flood type (C1) (C2) (C3) Widespread Widespread Rain 100 mm Localised Rain “HANDY” to flooded “GOOD” flood Then increases (kg DM/ha of useful feed) (kg DM/ha of useful feed) (kg DM/ha of useful feed) 95 “GOOD” flood Good Good floods are similar to handy floods, but cover a much higher C1, C3, C2 Flooded across most of 85 - 100% of to proportion of the floodplain (75% or more) and grow more feed per floodplains potential cattle Good 1200-2500 1500-3500 4500-8000 90 area than a handy flood. 80-100% inundation numbers 85 IF in 24-72 hrs Handy Handy floods occur when the water escapes from the gutters, C1, C3 Pushing out of gutters across 45 - 85% of Handy 750-1500 100-250 3500-6500 80 PRIOR, rains of (or useful) connecting up to form the large sheets of water. -

Using Genome-Wide Snps
CSIRO PUBLISHING Marine and Freshwater Research, 2019, 70, 857–869 https://doi.org/10.1071/MF18347 Phylogeography and species delimitation of Cherax destructor (Decapoda: Parastacidae) using genome-wide SNPs P. J. UnmackA,C, M. J. YoungA, B. GruberA, D. WhiteA, A. KilianB, X. ZhangA and A. GeorgesA AInstitute for Applied Ecology, University of Canberra, ACT 2601, Australia. BDiversity Arrays Technology Pty Ltd, University of Canberra, ACT 2601, Australia. CCorresponding author. Email: [email protected] Abstract. Cherax is a genus of 58 species of decapod crustaceans that are widespread across Australia and New Guinea. We use single-nucleotide polymorphisms (SNPs) to examine phylogeographic patterns in the most widespread species of Cherax, namely, C. destructor, and test the distinctiveness of one undescribed species, two C. destructor subspecies, previously proposed evolutionarily significant units, and management units. Both the phylogenetic analyses and the analysis of fixed allelic differences between populations support the current species-level taxonomy of C. setosus, C. depressus, C. dispar and C. destructor, the distinctiveness of C. destructor albidus and C. d. destructor and the existence of one undescribed species. The two populations of C. d. albidus from the Glenelg and Wimmera rivers were significantly distinct, with eight diagnostic differences (,1% fixed differences, null expectation is four fixed differences), but this low level of divergence is interpreted as within the range that might be expected of management units, that is, among allopatric populations of a single species or subspecies. A southern clade of C. d. destructor comprising the Murray River and its tributaries upstream from its confluence with the Darling River is genetically distinct from a northern clade comprising populations from the Lake Eyre Basin, the northern half of the Murray–Darling Basin (Darling River catchment) and the Lower Murray River below the Darling confluence. -

Land Zones of Queensland
P.R. Wilson and P.M. Taylor§, Queensland Herbarium, Department of Science, Information Technology, Innovation and the Arts. © The State of Queensland (Department of Science, Information Technology, Innovation and the Arts) 2012. Copyright inquiries should be addressed to <[email protected]> or the Department of Science, Information Technology, Innovation and the Arts, 111 George Street, Brisbane QLD 4000. Disclaimer This document has been prepared with all due diligence and care, based on the best available information at the time of publication. The department holds no responsibility for any errors or omissions within this document. Any decisions made by other parties based on this document are solely the responsibility of those parties. Information contained in this document is from a number of sources and, as such, does not necessarily represent government or departmental policy. If you need to access this document in a language other than English, please call the Translating and Interpreting Service (TIS National) on 131 450 and ask them to telephone Library Services on +61 7 3224 8412. This publication can be made available in an alternative format (e.g. large print or audiotape) on request for people with vision impairment; phone +61 7 3224 8412 or email <[email protected]>. ISBN: 978-1-920928-21-6 Citation This work may be cited as: Wilson, P.R. and Taylor, P.M. (2012) Land Zones of Queensland. Queensland Herbarium, Queensland Department of Science, Information Technology, Innovation and the Arts, Brisbane. 79 pp. Front Cover: Design by Will Smith Images – clockwise from top left: ancient sandstone formation in the Lawn Hill area of the North West Highlands bioregion – land zone 10 (D. -

Common Native Fish of the Lake Eyre Rivers Fact Sheet
To survive the long hot summers that characterise the Arid Lands region, native fish in the Lake Eyre Basin must be able to survive in isolated, often small pools. LAKE EYRE BASIN FISH Here they have to cope with very high temperatures, low oxygen and extremely ARE UNIQUE WITH MANY high salinities (often saltier than sea-water). SPECIES FOUND ONLY They must also be able to take advantage of periods of flooding to rebuild AROUND LAKE EYRE population levels and recolonise newly filled habitats. Around 20 native fish species are found in the Lake Eyre Basin – read on to find out more about some of the more common ones… RECOVERING FROM THE DROUGHT... Research by the South Australian recover following rains others can Arid Lands NRM Board and South take many years to recolonise Australian Research and Development catchments and rebuild populations. Institute has revealed that it takes Large floods may be required before many years for all species of fish to some species return to their former recover from drought. habitats. The team tracked the recolonisation At present some species remain in of the Neales River catchment after very few waterholes and are at high recent dry conditions reduced the risk of becoming locally extinct. river into only a few pools. The research team hopes to find out Although there have been no large exactly what conditions each fish species floods since the drought, since 2006 needs to survive and recover from large there have been short periods of disturbances such as drought, or from ‘within channel’ flow during relatively impacts that humans may impose in mild wet seasons. -

Wetland Mapping, Channel Country Bioregion
Department for Environment and Heritage Wetland Mapping Channel Country bioregion, South Australia www.environment.sa.gov.au Wetland Mapping Channel Country bioregion, South Australia. P. Wainwright1, Y. Tunn2, D. Gibson2 and J. Cameron2 1 Land Management Branch, Department for Environment and Heritage, SA. 2 Environmental Information, Department for Environment and Heritage, SA TABLE OF CONTENTS PART ONE - GENERAL DISCUSSION EXECUTIVE SUMMARY………………………………………………………………………………………………….…….….…1 1.0 INTRODUCTION………………………………………………………………………………………………………………......2 1.1.Cooper Creek.......................................................................................................................................................2 1.2. Diamantina River.................................................................................................................................................2 1.3. Georgina River ....................................................................................................................................................2 1.4. Why are the wetlands in the Channel Country significant?.................................................................................4 1.5. Wetland definition................................................................................................................................................5 1.6. Wetland characteristics .......................................................................................................................................5 1.7. Important