Response Strategies Against Meningitis Epidemics After
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
UNHCR Niger Operation UNHCR Database
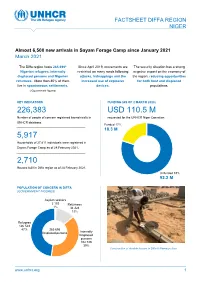
FACTSHEET DIFFA REGION NIGER Almost 6,500 new arrivals in Sayam Forage Camp since January 2021 March 2021 NNNovember The Diffa region hosts 265,696* Since April 2019, movements are The security situation has a strong Nigerian refugees, internally restricted on many roads following negative impact on the economy of displaced persons and Nigerien attacks, kidnappings and the the region, reducing opportunities returnees. More than 80% of them increased use of explosive for both host and displaced live in spontaneous settlements. devices. populations. (*Government figures) KEY INDICATORS FUNDING (AS OF 2 MARCH 2020) 226,383 USD 110.5 M Number of people of concern registered biometrically in requested for the UNHCR Niger Operation UNHCR database. Funded 17% 18.3 M 5,917 Households of 27,811 individuals were registered in Sayam Forage Camp as of 28 February 2021. 2,710 Houses built in Diffa region as of 28 February 2021. Unfunded 83% 92.2 M the UNHCR Niger Operation POPULATION OF CONCERN IN DIFFA (GOVERNMENT FIGURES) Asylum seekers 2 103 Returnees 1% 34 324 13% Refugees 126 543 47% 265 696 Displaced persons Internally Displaced persons 102 726 39% Construction of durable houses in Diffa © Ramatou Issa www.unhcr.org 1 OPERATIONAL UPDATE > Niger - Diffa / March 2021 Operation Strategy The key pillars of the UNHCR strategy for the Diffa region are: ■ Ensure institutional resilience through capacity development and support to the authorities (locally elected and administrative authorities) in the framework of the Niger decentralisation process. ■ Strengthen the out of camp policy around the urbanisation program through sustainable interventions and dynamic partnerships including with the World Bank. -

NIGER: Carte Administrative NIGER - Carte Administrative
NIGER - Carte Administrative NIGER: Carte administrative Awbari (Ubari) Madrusah Légende DJANET Tajarhi /" Capital Illizi Murzuq L I B Y E !. Chef lieu de région ! Chef lieu de département Frontières Route Principale Adrar Route secondaire A L G É R I E Fleuve Niger Tamanghasset Lit du lac Tchad Régions Agadez Timbuktu Borkou-Ennedi-Tibesti Diffa BARDAI-ZOUGRA(MIL) Dosso Maradi Niamey ZOUAR TESSALIT Tahoua Assamaka Tillabery Zinder IN GUEZZAM Kidal IFEROUANE DIRKOU ARLIT ! BILMA ! Timbuktu KIDAL GOUGARAM FACHI DANNAT TIMIA M A L I 0 100 200 300 kms TABELOT TCHIROZERINE N I G E R ! Map Doc Name: AGADEZ OCHA_SitMap_Niger !. GLIDE Number: 16032013 TASSARA INGALL Creation Date: 31 Août 2013 Projection/Datum: GCS/WGS 84 Gao Web Resources: www.unocha..org/niger GAO Nominal Scale at A3 paper size: 1: 5 000 000 TILLIA TCHINTABARADEN MENAKA ! Map data source(s): Timbuktu TAMAYA RENACOM, ARC, OCHA Niger ADARBISNAT ABALAK Disclaimers: KAOU ! TENIHIYA The designations employed and the presentation of material AKOUBOUNOU N'GOURTI I T C H A D on this map do not imply the expression of any opinion BERMO INATES TAKANAMATAFFALABARMOU TASKER whatsoever on the part of the Secretariat of the United Nations BANIBANGOU AZEY GADABEDJI TANOUT concerning the legal status of any country, territory, city or area ABALA MAIDAGI TAHOUA Mopti ! or of its authorities, or concerning the delimitation of its YATAKALA SANAM TEBARAM !. Kanem WANZERBE AYOROU BAMBAYE KEITA MANGAIZE KALFO!U AZAGORGOULA TAMBAO DOLBEL BAGAROUA TABOTAKI TARKA BANKILARE DESSA DAKORO TAGRISS OLLELEWA -

Resident / Humanitarian Coordinator Report on the Use of Cerf Funds Niger Rapid Response Conflict-Related Displacement
RESIDENT / HUMANITARIAN COORDINATOR REPORT ON THE USE OF CERF FUNDS NIGER RAPID RESPONSE CONFLICT-RELATED DISPLACEMENT RESIDENT/HUMANITARIAN COORDINATOR Mr. Fodé Ndiaye REPORTING PROCESS AND CONSULTATION SUMMARY a. Please indicate when the After Action Review (AAR) was conducted and who participated. Since the implementation of the response started, OCHA has regularly asked partners to update a matrix related to the state of implementation of activities, as well as geographical location of activities. On February 26, CERF-focal points from all agencies concerned met to kick off the reporting process and establish a framework. This was followed up by submission of individual projects and input in the following weeks, as well as consolidation and consultation in terms of the draft for the report. b. Please confirm that the Resident Coordinator and/or Humanitarian Coordinator (RC/HC) Report was discussed in the Humanitarian and/or UN Country Team and by cluster/sector coordinators as outlined in the guidelines. YES NO c. Was the final version of the RC/HC Report shared for review with in-country stakeholders as recommended in the guidelines (i.e. the CERF recipient agencies and their implementing partners, cluster/sector coordinators and members and relevant government counterparts)? YES NO The CERF Report has been shared with Cluster Coordinator and recipient agencies. 2 I. HUMANITARIAN CONTEXT TABLE 1: EMERGENCY ALLOCATION OVERVIEW (US$) Total amount required for the humanitarian response: 53,047,888 Source Amount CERF 5,181,281 Breakdown -

NIGER Community Action Program and Community-Based Integrated Ecosystem Management Project Phase I and II
NIGER Community Action Program and Community-Based Integrated Ecosystem Management Project Phase I and II Report No. 155367 DECEMBER 31, 2020 © 2021 International Bank for Reconstruction and Development / The World Bank 1818 H Street NW Washington DC 20433 Telephone: 202-473-1000 Internet: www.worldbank.org Attribution—Please cite the work as follows: World Bank. 2021. Niger—Community Action Program and Community-Based Integrated Ecosystem Management Project. Independent Evaluation Group, Project Performance Assessment Report 155367. Washington, DC: World Bank. This work is a product of the staff of The World Bank with external contributions. The findings, interpretations, and conclusions expressed in this work do not necessarily reflect the views of The World Bank, its Board of Executive Directors, or the governments they represent. The World Bank does not guarantee the accuracy of the data included in this work. The boundaries, colors, denominations, and other information shown on any map in this work do not imply any judgment on the part of The World Bank concerning the legal status of any territory or the endorsement or acceptance of such boundaries. RIGHTS AND PERMISSIONS The material in this work is subject to copyright. Because The World Bank encourages dissemination of its knowledge, this work may be reproduced, in whole or in part, for noncommercial purposes as long as full attribution to this work is given. Any queries on rights and licenses, including subsidiary rights, should be addressed to World Bank Publications, The World Bank Group, 1818 H Street NW, Washington, DC 20433, USA; fax: 202-522-2625; e-mail: [email protected]. -

Niger Food Security Brief
NIGER FOOD SECURITY BRIEF MAY 2014 Niger Food Security Brief This publication was prepared by Meredith Sisa under the United States Agency for International Development Famine Early Warning Systems Network (FEWS NET) Indefinite Quantity Contract, AID-OAA-I-12-00006. The author’s views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States Government. Photo credit: Peter Thomas, FEWS NET Page 2 Niger Food Security Brief Introduction Over the last three decades, FEWS NET has steadily built a core set of integrated materials on livelihoods, household vulnerability, nutrition, trade, and agro- climatology through fieldwork and secondary data collection and research. FEWS NET also looks beyond the immediate context to understand the broader context and the underlying causes of food insecurity. This Food Security Brief draws on FEWS NET research and an array of other sources to provide an overview of the food security context and the main determinants of chronic and acute food insecurity, and highlight areas or livelihood zones at most risk of food insecurity. It is a starting ABOUT point for anyone seeking a deep understanding of the range of factors influencing food security in Niger. F E W S N E T The brief is organized around the FEWS NET Household Livelihoods Analytical Created in response to Framework (Figure 1), which looks at underlying and proximate causes of food the 1984 famines in insecurity as a means to inform outcomes at the regional and household levels. At East and West Africa, the core of this analysis is an understanding of hazards and their magnitude and the Famine Early extent, household vulnerability to hazards, and coping capacity in response. -

UNFPA–UNICEF Global Programme to End Child Marriage
NIGER COUNTRY PROFILE UNFPA–UNICEF Global Programme to End Child Marriage Dejongh © UNICEF/UN0317837/Frank NIGER COUNTRY PROFILE 0% Niger is home to 5 10% million child brides. 20% Of these, 1.9 million 30% married before age 15. 40% 50% Source: UNICEF global databases, 2020. Demographic data are 5 million 60% from United Nations, Department Married before age 18 of Economic and Social Affairs, 70% Population Division (2019). World Population Prospects 2019, Online 80% Edition. Rev. 1. 90% Notes: For details on the calculation of girls and women married in 100% childhood, see: United Nations Children’s Fund, Child Marriage: Latest trends and future prospects, Percentage of women aged 20 to 24 years who were first married UNICEF, New York, 2018. Estimates or in union before age 18 refer to population year 2019. Values below 2 million are rounded to 1.9 million Note: This map is stylized and not to scale. It does not reflect a position by UNFPA the nearest hundred thousand; those or UNICEF on the legal status of any country or area or the delimitation of any Married before age 15 above 2 million are rounded to the frontiers. Source for child marriage prevalence data is the Niger Demographic and nearest million. Health Survey 2012. Before age 15 Before age 18 Percentage of women 100 aged 20 to 24 years 90 84 who were first married 80 76 or in union before age 15 and before age 18 70 60 Source: Niger Demographic and Health Survey 2012 50 Note: This trend analysis is based on the prevalence of child marriage 40 across age cohorts, as measured in 34 the latest available survey. -
Niger 2020 Human Rights Report
NIGER 2020 HUMAN RIGHTS REPORT EXECUTIVE SUMMARY Niger is a multiparty republic. In the first round of the presidential elections on December 27, Mohamed Bazoum of the ruling coalition finished first with 39.3 percent of the vote. Opposition candidate Mahamane Ousman finished second with 16.9 percent. A second round between the two candidates was scheduled for February 21, 2021. President Mahamadou Issoufou, who won a second term in 2016, was expected to continue in office until the second round was concluded and the winner sworn into office. International and domestic observers found the first round of the presidential election to be peaceful, free, and fair. In parallel legislative elections also conducted on December 27, the ruling coalition preliminarily won 80 of 171 seats, and various opposition parties divided the rest, with several contests still to be decided. International and local observers found the legislative elections to be equally peaceful, free, and fair. The National Police, under the Ministry of Interior, Public Security, Decentralization, and Customary and Religious Affairs (Ministry of Interior), is responsible for urban law enforcement. The Gendarmerie, under the Ministry of National Defense, has primary responsibility for rural security. The National Guard, also under the Ministry of Interior, is responsible for domestic security and the protection of high-level officials and government buildings. The armed forces, under the Ministry of National Defense, are responsible for external security and, in some parts of the country, for internal security. Every 90 days the parliament reviews the state of emergency declaration in effect in the Diffa Region and in parts of Tahoua and Tillabery Regions. -
Niger Page 1 of 27
2009 Human Rights Report: Niger Page 1 of 27 Home » Under Secretary for Democracy and Global Affairs » Bureau of Democracy, Human Rights, and Labor » Releases » Human Rights Reports » 2009 Country Reports on Human Rights Practices » Africa » Niger 2009 Human Rights Report: Niger BUREAU OF DEMOCRACY, HUMAN RIGHTS, AND LABOR 2009 Country Reports on Human Rights Practices March 11, 2010 Niger is a republic that restored its multiparty system in 1999 following coups in 1996 and 1999; it has a population estimated at 15.4 million. In 2004 voters elected Mamadou Tandja to a second five-year presidential term in an election that international observers deemed generally free and fair. The ruling coalition of the National Movement for the Development of Society (MNSD) and the Democratic and Social Convention (CDS), joined by four other parties, won a majority of national assembly seats. President Tandja's second--and final, due to constitutional limits--five-year term was due to expire on December 22, 2009; however, he organized a controversial referendum that established the Sixth Republic and allowed him to remain in office for three additional years and that eliminated the term-limits provision, although this provision was specifically prohibited from revision in the 1999 constitution. To consolidate the power needed to approve these changes, President Tandja dissolved the National Assembly and the Constitutional Court, modified the electoral code, restricted basic freedoms, curtailed press freedom, and granted himself emergency powers to rule by decree and executive order. In 2007 the Tuareg rebel group Nigerien Movement for Justice (MNJ) launched a series of attacks against military and strategic installations in the north. -

Assessment of Chronic Food Insecurity in Niger
Assessment of Chronic Food Insecurity in Niger Analysis Coordination March 2019 Assessment of Chronic Food Insecurity in Niger 2019 About FEWS NET Created in response to the 1984 famines in East and West Africa, the Famine Early Warning Systems Network (FEWS NET) provides early warning and integrated, forward-looking analysis of the many factors that contribute to food insecurity. FEWS NET aims to inform decision makers and contribute to their emergency response planning; support partners in conducting early warning analysis and forecasting; and provide technical assistance to partner-led initiatives. To learn more about the FEWS NET project, please visit www.fews.net. Acknowledgements This publication was prepared under the United States Agency for International Development Famine Early Warning Systems Network (FEWS NET) Indefinite Quantity Contract, AID-OAA-I-12-00006. The author’s views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States Government. Recommended Citation FEWS NET. 2019. Assessment of Chronic Food Insecurity in Niger. Washington, DC: FEWS NET. Famine Early Warning Systems Network ii Assessment of Chronic Food Insecurity in Niger 2019 Table of Contents Executive Summary ..................................................................................................................................................................... 1 Background ............................................................................................................................................................................. -

Insecurity, Terrorism, and Arms Trafficking in Niger
Small Arms Survey Maison de la Paix Report Chemin Eugène-Rigot 2E January 1202 Geneva 2018 Switzerland t +41 22 908 5777 f +41 22 732 2738 e [email protected] At the Crossroads of Sahelian Conflicts Sahelian of the Crossroads At About the Small Arms Survey The Small Arms Survey is a global centre of excellence whose mandate is to generate impar- tial, evidence-based, and policy-relevant knowledge on all aspects of small arms and armed AT THE CROSSROADS OF violence. It is the principal international source of expertise, information, and analysis on small arms and armed violence issues, and acts as a resource for governments, policy- makers, researchers, and civil society. It is located in Geneva, Switzerland, at the Graduate SAHELIAN CONFLICTS Institute of International and Development Studies. The Survey has an international staff with expertise in security studies, political science, Insecurity, Terrorism, and law, economics, development studies, sociology, and criminology, and collaborates with a network of researchers, partner institutions, non-governmental organizations, and govern- Arms Trafficking in Niger ments in more than 50 countries. For more information, please visit: www.smallarmssurvey.org. Savannah de Tessières A publication of the Small Arms Survey/SANA project, with the support of the Netherlands Ministry of Foreign Affairs, Global Affairs Canada, and the Swiss Federal Department of Foreign Affairs A T THE CROSSROADS OF SAHELian CONFLictS Insecurity, Terrorism, and Arms Trafficking in Niger Savannah de Tessières A publication of the Small Arms Survey/SANA project, with the support of the Netherlands Min. of Foreign Affairs, Global Affairs Canada, & the Swiss Federal Dept. -

Usaid Humanitarian Assistance to Niger for Malnutrition and Food Insecurity in Fy 2010
USAID HUMANITARIAN ASSISTANCE TO NIGER FOR MALNUTRITION AND FOOD INSECURITY IN FY 2010 0°KEY 2° 4° 6° 8° 10° 12° 14° 16° USAID/OFDA USAID/FFP 0 200 400 mi LIBYA FOR C IN MA I TI PH O A N Agriculture and Food Security Tamanrasset 0 200 400 600 km R U A G NIGER N O I T E C Economic Recovery and Market Systems G U S A A D Emergency Food Assistance ID F y Madama /DCHA/O 22° Food Vouchers Affected Areas b Humanitarian Air Service OCHA B UNHAS Humanitarian Coordination and b B Information Management UNICEF Djado Zouar 5 Local Food Procurement and Distribution CRS, CARE, & HKI Consortium y Logistics and Relief Commodities ALGERIA WFP 5 Séguedine a20° y 20° Nutrition Water, Sanitation, and Hygiene J I-n-Guezzâm AGADEZ 07/14/10 Assamakka Mercy Corps CJ Dirkou Arlit Bilma MALI AGADEZ 18° Timia Fachi 18° TILLABÉRI CRS Teguidda-n- A Tessoumt Mercy Corps 5 Oxfam/GB Tassara 7 Agadez DIFFA AC Ingal VSF/B HKI Gao AC WFP C TAHOUA CPI y 16° 16° WV Ménaka Concern N C ZINDER ig e DIFFA r CRS FAO A Aderbissinat CRS Termit- Mercy Corps A Kaoboul 5 Njourti TAHOUA FAO A Bani Bangou HKI CHAD Yatakala Tahoua Abalak Ayorou Tânout IFRC Bagaroua Keïta Bankilaré CPI y Tillabéry Ouallam Filingué Dakoro ZINDER Illéla Bouza Nguigmi Mao 14° Téra 14° TILLABÉRI MARADI Gouré Baléyara Madaoua Lake Chad Dargol Dogondoutchi Tessaoua Niamey Birnin Zinder Maradi Bol 7 DOSSO Konni Maïné Diffa BURKINA Birnin DOSSO ima Yob R Soroa u Say Gaouré CRS ug FASO A MARADI d Niamey Dosso a Sokoto Magaria m HKI Nguru Komadugu o C CRS K AM IFRC Katsina A E o S R t ok a La Tapoa IFRCo o d O Kantchari k t d O Mercy o o Oxfam/GBKaura a a Dioundiou S AC an g N Corps C Namoda G N Birnin Kebbi gu SC/UK u N C d ig Zamfara Original Map Courtesy of the UN CartographicN'Djamena Section e Gusau ia a 12° r ej m C 12° Diapaga Gaya WV ad o NIGERIA Fada- Kano H K The boundaries and names used on thish map do a r Ngourma Dutse not imply official endorsement or acceptancei 0° 2° BENIN 4°Ka 6° 8° 10° 12°by the U.S. -

Agadez, Niger
Mayors Dialogue on Growth and Solidarity City profile: Agadez, Niger Population: 137,354 (2017 ) GDP per capita: $555 (2019, national) Major industries: trade and logistics, livestock, agriculture Percentage of migrants: 1% (2017, national) Mayor’s name: Dr Boukari Mamane | Next election date: 2021 Socioeconomic profile Migration profile Agadez is the fifth largest city in Niger, capital Agadez is home to a large number of internal rural of both the Agadez Region and Aïr, a traditional migrants who moved to the city in the 1970s and Tuareg–Berber federation (Institut National de la 1980s to escape severe droughts. Changes in rainfall Statistique, 2014). The city is geographically dispersed causing both drought and flash floods continue and is home to 137,354 individuals over an area to displace people across Niger (121,000 in 2019) of 213 km2 (Institut National de la Statistique du (Internal Displacement Monitoring Centre, n.d.). In Niger, 2020a). Since 2000, the population has been terms of international migration, Niger is home to growing by 3.17% annually (Institut National de la 295,600 foreign-born residents, mostly from nearby Statistique du Niger, 2019). A substantial proportion countries (Nigeria, Mali and Burkina Faso) (EU of Agadez residents are nomadic or semi-nomadic Commission, 2019). Out of Niger’s population of people (Molenaar et al., 2017). The city’s population around 21.5 million, this implies a national migrant is younger than the Nigerien average, with 40% population of around 1% (EU Commission, 2019). between the ages of 15 and 39 (Institut National de la In 2017, there were also 165,700 refugees and 300 Statistique du Niger, 2020b).