Gemin Proteins Are Required for Efficient Assembly of Sm-Class Ribonucleoproteins
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DEAD-Box RNA Helicases in Cell Cycle Control and Clinical Therapy
cells Review DEAD-Box RNA Helicases in Cell Cycle Control and Clinical Therapy Lu Zhang 1,2 and Xiaogang Li 2,3,* 1 Department of Nephrology, Renmin Hospital of Wuhan University, Wuhan 430060, China; [email protected] 2 Department of Internal Medicine, Mayo Clinic, 200 1st Street, SW, Rochester, MN 55905, USA 3 Department of Biochemistry and Molecular Biology, Mayo Clinic, 200 1st Street, SW, Rochester, MN 55905, USA * Correspondence: [email protected]; Tel.: +1-507-266-0110 Abstract: Cell cycle is regulated through numerous signaling pathways that determine whether cells will proliferate, remain quiescent, arrest, or undergo apoptosis. Abnormal cell cycle regula- tion has been linked to many diseases. Thus, there is an urgent need to understand the diverse molecular mechanisms of how the cell cycle is controlled. RNA helicases constitute a large family of proteins with functions in all aspects of RNA metabolism, including unwinding or annealing of RNA molecules to regulate pre-mRNA, rRNA and miRNA processing, clamping protein complexes on RNA, or remodeling ribonucleoprotein complexes, to regulate gene expression. RNA helicases also regulate the activity of specific proteins through direct interaction. Abnormal expression of RNA helicases has been associated with different diseases, including cancer, neurological disorders, aging, and autosomal dominant polycystic kidney disease (ADPKD) via regulation of a diverse range of cellular processes such as cell proliferation, cell cycle arrest, and apoptosis. Recent studies showed that RNA helicases participate in the regulation of the cell cycle progression at each cell cycle phase, including G1-S transition, S phase, G2-M transition, mitosis, and cytokinesis. -

Supplementary Table S1. Correlation Between the Mutant P53-Interacting Partners and PTTG3P, PTTG1 and PTTG2, Based on Data from Starbase V3.0 Database
Supplementary Table S1. Correlation between the mutant p53-interacting partners and PTTG3P, PTTG1 and PTTG2, based on data from StarBase v3.0 database. PTTG3P PTTG1 PTTG2 Gene ID Coefficient-R p-value Coefficient-R p-value Coefficient-R p-value NF-YA ENSG00000001167 −0.077 8.59e-2 −0.210 2.09e-6 −0.122 6.23e-3 NF-YB ENSG00000120837 0.176 7.12e-5 0.227 2.82e-7 0.094 3.59e-2 NF-YC ENSG00000066136 0.124 5.45e-3 0.124 5.40e-3 0.051 2.51e-1 Sp1 ENSG00000185591 −0.014 7.50e-1 −0.201 5.82e-6 −0.072 1.07e-1 Ets-1 ENSG00000134954 −0.096 3.14e-2 −0.257 4.83e-9 0.034 4.46e-1 VDR ENSG00000111424 −0.091 4.10e-2 −0.216 1.03e-6 0.014 7.48e-1 SREBP-2 ENSG00000198911 −0.064 1.53e-1 −0.147 9.27e-4 −0.073 1.01e-1 TopBP1 ENSG00000163781 0.067 1.36e-1 0.051 2.57e-1 −0.020 6.57e-1 Pin1 ENSG00000127445 0.250 1.40e-8 0.571 9.56e-45 0.187 2.52e-5 MRE11 ENSG00000020922 0.063 1.56e-1 −0.007 8.81e-1 −0.024 5.93e-1 PML ENSG00000140464 0.072 1.05e-1 0.217 9.36e-7 0.166 1.85e-4 p63 ENSG00000073282 −0.120 7.04e-3 −0.283 1.08e-10 −0.198 7.71e-6 p73 ENSG00000078900 0.104 2.03e-2 0.258 4.67e-9 0.097 3.02e-2 Supplementary Table S2. -

Lorena Novoa-Aponte
A Strain: fl/fl ∆hep Strain: fl/fl ∆hep AnalysisAAV: ofLuc the ironLuc chaperonePCBP1-WT PCBP1PCBP1-∆Fe interactome:PCBP1-∆RNA PCBP1 variant intersection of DNA repair and iron traffickingAAV: Luc Luc WT ∆Fe ∆RNA PCBP1 −40 Lorena Novoa-Aponte a*, Sarju J. Patel a, Olga Protchenko a, James Wohlschlegel b, Caroline C. Philpott a −40 PCBP1, IHC PCBP1, GAPDH - a Genetics and Metabolism Section,a NIDDK, NIH, Bethesda, MD, USA. b Department of Biological Chemistry, UCLA, Los Angeles, CA, USA C.C. Philpott, et al. *[email protected] 80 B P = 0.0262 60 40 1. Iron is essential but toxic 2. Increased DNA damage in cells lacking PCBP1 ns H&E ? + 20 C.C. Philpott, et al. Iron is used as an essential cofactor by several enzymes involved in DNA ⦿ Increased TUNEL in cells and tissue livers from mice lacking PCBP1. ? replication and repair. However, unchaperoned iron promotes redox ⦿ PCBP1 binds both, iron and single-stranded nucleicTG, nmol/mg protein 0 acids. stress that may affect DNA stability. Strain: Δhep Δhep Δhep ? ⦿ The iron binding activity of PCBP1 controls suppressionAAV: of DNA damage C WT ∆Fe ∆RNA PCBP1 variant ? Iron storage HEK293 cells Mouse Liver ? A 8 siNT+P1var 100 ? A 8 var siPCBP1 2 ? Mammals use the iron siNT+P1 ns var Fe-S cluster assembly 6 siPCBP1+P1siPCBP1 80 ADI1 TUNEL chaperone PCBP1 to var ? 6 siPCBP1+P1 metalate several iron- 60 = 0.0468 = 0.0354 = 0.0465 cell / mm population P P P population + dependent enzymes + 4 + 4 ? 40 Degradation of HIF1α Fig. 3. Iron chaperone-mediated handling of cytosolic labile iron pool. -

Newfound Coding Potential of Transcripts Unveils Missing Members Of
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.02.406710; this version posted December 3, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Newfound coding potential of transcripts unveils missing members of 2 human protein communities 3 4 Sebastien Leblanc1,2, Marie A Brunet1,2, Jean-François Jacques1,2, Amina M Lekehal1,2, Andréa 5 Duclos1, Alexia Tremblay1, Alexis Bruggeman-Gascon1, Sondos Samandi1,2, Mylène Brunelle1,2, 6 Alan A Cohen3, Michelle S Scott1, Xavier Roucou1,2,* 7 1Department of Biochemistry and Functional Genomics, Université de Sherbrooke, Sherbrooke, 8 Quebec, Canada. 9 2 PROTEO, Quebec Network for Research on Protein Function, Structure, and Engineering. 10 3Department of Family Medicine, Université de Sherbrooke, Sherbrooke, Quebec, Canada. 11 12 *Corresponding author: Tel. (819) 821-8000x72240; E-Mail: [email protected] 13 14 15 Running title: Alternative proteins in communities 16 17 Keywords: alternative proteins, protein network, protein-protein interactions, pseudogenes, 18 affinity purification-mass spectrometry 19 20 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.12.02.406710; this version posted December 3, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 21 Abstract 22 23 Recent proteogenomic approaches have led to the discovery that regions of the transcriptome 24 previously annotated as non-coding regions (i.e. -

Gemin4 Is an Essential Gene in Mice, and Its Overexpression in Human Cells Causes Relocalization of the SMN Complex to the Nucleoplasm Ingo D
© 2018. Published by The Company of Biologists Ltd | Biology Open (2018) 7, bio032409. doi:10.1242/bio.032409 RESEARCH ARTICLE Gemin4 is an essential gene in mice, and its overexpression in human cells causes relocalization of the SMN complex to the nucleoplasm Ingo D. Meier1,*,§, Michael P. Walker1,2,‡,§ and A. Gregory Matera¶ ABSTRACT nuclear ribonucleoproteins (snRNPs). Each of these snRNPs Gemin4 is a member of the Survival Motor Neuron (SMN) protein contains a common set of seven RNA binding factors, called Sm complex, which is responsible for the assembly and maturation of Sm- proteins, that forms a heptameric ring around the snRNA, known as class small nuclear ribonucleoproteins (snRNPs). In metazoa, Sm the Sm core. Biogenesis of the Sm core is carried out by another snRNPs are assembled in the cytoplasm and subsequently imported macromolecular assemblage called the Survival Motor Neuron into the nucleus. We previously showed that the SMN complex is (SMN) complex, consisting of at least nine proteins (Gemins 2-8, required for snRNP import in vitro, although it remains unclear which unrip and SMN) (reviewed in Battle et al., 2006a; Matera et al., specific components direct this process. Here, we report that Gemin4 2007; Matera and Wang, 2014). overexpression drives SMN and the other Gemin proteins from the Following RNA polymerase II-mediated transcription in the cytoplasm into the nucleus. Moreover, it disrupts the subnuclear nucleus, pre-snRNAs are exported to the cytoplasm for assembly localization of the Cajal body marker protein, coilin, in a dose- into stable RNP particles (Jarmolowski et al., 1994; Ohno et al., dependent manner. -

Inhibition of the MID1 Protein Complex
Matthes et al. Cell Death Discovery (2018) 4:4 DOI 10.1038/s41420-017-0003-8 Cell Death Discovery ARTICLE Open Access Inhibition of the MID1 protein complex: a novel approach targeting APP protein synthesis Frank Matthes1,MoritzM.Hettich1, Judith Schilling1, Diana Flores-Dominguez1, Nelli Blank1, Thomas Wiglenda2, Alexander Buntru2,HannaWolf1, Stephanie Weber1,InaVorberg 1, Alina Dagane2, Gunnar Dittmar2,3,ErichWanker2, Dan Ehninger1 and Sybille Krauss1 Abstract Alzheimer’s disease (AD) is characterized by two neuropathological hallmarks: senile plaques, which are composed of amyloid-β (Aβ) peptides, and neurofibrillary tangles, which are composed of hyperphosphorylated tau protein. Aβ peptides are derived from sequential proteolytic cleavage of the amyloid precursor protein (APP). In this study, we identified a so far unknown mode of regulation of APP protein synthesis involving the MID1 protein complex: MID1 binds to and regulates the translation of APP mRNA. The underlying mode of action of MID1 involves the mTOR pathway. Thus, inhibition of the MID1 complex reduces the APP protein level in cultures of primary neurons. Based on this, we used one compound that we discovered previously to interfere with the MID1 complex, metformin, for in vivo experiments. Indeed, long-term treatment with metformin decreased APP protein expression levels and consequently Aβ in an AD mouse model. Importantly, we have initiated the metformin treatment late in life, at a time-point where mice were in an already progressed state of the disease, and could observe an improved behavioral phenotype. These 1234567890 1234567890 findings together with our previous observation, showing that inhibition of the MID1 complex by metformin also decreases tau phosphorylation, make the MID1 complex a particularly interesting drug target for treating AD. -

The Genetic Program of Pancreatic Beta-Cell Replication in Vivo
Page 1 of 65 Diabetes The genetic program of pancreatic beta-cell replication in vivo Agnes Klochendler1, Inbal Caspi2, Noa Corem1, Maya Moran3, Oriel Friedlich1, Sharona Elgavish4, Yuval Nevo4, Aharon Helman1, Benjamin Glaser5, Amir Eden3, Shalev Itzkovitz2, Yuval Dor1,* 1Department of Developmental Biology and Cancer Research, The Institute for Medical Research Israel-Canada, The Hebrew University-Hadassah Medical School, Jerusalem 91120, Israel 2Department of Molecular Cell Biology, Weizmann Institute of Science, Rehovot, Israel. 3Department of Cell and Developmental Biology, The Silberman Institute of Life Sciences, The Hebrew University of Jerusalem, Jerusalem 91904, Israel 4Info-CORE, Bioinformatics Unit of the I-CORE Computation Center, The Hebrew University and Hadassah, The Institute for Medical Research Israel- Canada, The Hebrew University-Hadassah Medical School, Jerusalem 91120, Israel 5Endocrinology and Metabolism Service, Department of Internal Medicine, Hadassah-Hebrew University Medical Center, Jerusalem 91120, Israel *Correspondence: [email protected] Running title: The genetic program of pancreatic β-cell replication 1 Diabetes Publish Ahead of Print, published online March 18, 2016 Diabetes Page 2 of 65 Abstract The molecular program underlying infrequent replication of pancreatic beta- cells remains largely inaccessible. Using transgenic mice expressing GFP in cycling cells we sorted live, replicating beta-cells and determined their transcriptome. Replicating beta-cells upregulate hundreds of proliferation- related genes, along with many novel putative cell cycle components. Strikingly, genes involved in beta-cell functions, namely glucose sensing and insulin secretion were repressed. Further studies using single molecule RNA in situ hybridization revealed that in fact, replicating beta-cells double the amount of RNA for most genes, but this upregulation excludes genes involved in beta-cell function. -

DDX20 Antibody Purified Mouse Monoclonal Antibody Catalog # Ao2157a
10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 DDX20 Antibody Purified Mouse Monoclonal Antibody Catalog # AO2157a Specification DDX20 Antibody - Product Information Application E, WB, IF, FC Primary Accession Q9UHI6 Reactivity Human, Rat Host Mouse Clonality Monoclonal Isotype IgG1 Calculated MW 92.2kDa KDa Description DEAD box proteins, characterized by the conserved motif Asp-Glu-Ala-Asp (DEAD), are putative RNA helicases. They are implicated in a number of cellular processes involving alteration of RNA secondary structure such as translation initiation, nuclear and mitochondrial splicing, and ribosome and spliceosome assembly. Based on their distribution patterns, some members of this family are believed to be involved in embryogenesis, spermatogenesis, and cellular growth and division. This gene encodes a DEAD box protein, which has an ATPase activity and is a component of the survival of motor neurons (SMN) complex. This protein interacts directly with SMN, the spinal muscular atrophy gene product, and may play a catalytic role in the function of the SMN complex on RNPs. Immunogen Purified recombinant fragment of human DDX20 (AA: 725-824) expressed in E. Coli. Formulation Purified antibody in PBS with 0.05% sodium azide DDX20 Antibody - Additional Information Gene ID 11218 Other Names Probable ATP-dependent RNA helicase DDX20, 3.6.4.13, Component of gems 3, Page 1/3 10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 DEAD box protein 20, DEAD box protein DP 103, Gemin-3, DDX20, DP103, GEMIN3 Dilution E~~1/10000 WB~~1/500 - 1/2000 IF~~1/200 - 1/1000 FC~~1/200 - 1/400 Storage Maintain refrigerated at 2-8°C for up to 6 months. -

The Combined Detection of Amphiregulin, Cyclin A1 and DDX20/Gemin3 Expression Predicts Aggressive Forms of Oral Squamous Cell Carcinoma
British Journal of Cancer www.nature.com/bjc ARTICLE OPEN Molecular Diagnostics The combined detection of Amphiregulin, Cyclin A1 and DDX20/Gemin3 expression predicts aggressive forms of oral squamous cell carcinoma 1 2 3 1 1 1 1 Ekaterina Bourova-Flin✉, Samira Derakhshan , Afsaneh✉ Goudarzi , Tao Wang , Anne-Laure Vitte , Florent Chuffart , Saadi Khochbin , Sophie Rousseaux 1 and Pouyan Aminishakib 2,4 © The Author(s) 2021 BACKGROUND: Large-scale genetic and epigenetic deregulations enable cancer cells to ectopically activate tissue-specific expression programmes. A specifically designed strategy was applied to oral squamous cell carcinomas (OSCC) in order to detect ectopic gene activations and develop a prognostic stratification test. METHODS: A dedicated original prognosis biomarker discovery approach was implemented using genome-wide transcriptomic data of OSCC, including training and validation cohorts. Abnormal expressions of silent genes were systematically detected, correlated with survival probabilities and evaluated as predictive biomarkers. The resulting stratification test was confirmed in an independent cohort using immunohistochemistry. RESULTS: A specific gene expression signature, including a combination of three genes, AREG, CCNA1 and DDX20, was found associated with high-risk OSCC in univariate and multivariate analyses. It was translated into an immunohistochemistry-based test, which successfully stratified patients of our own independent cohort. DISCUSSION: The exploration of the whole gene expression profile characterising -

DEAH)/RNA Helicase a Helicases Sense Microbial DNA in Human Plasmacytoid Dendritic Cells
Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells Taeil Kima, Shwetha Pazhoora, Musheng Baoa, Zhiqiang Zhanga, Shino Hanabuchia, Valeria Facchinettia, Laura Bovera, Joel Plumasb, Laurence Chaperotb, Jun Qinc, and Yong-Jun Liua,1 aDepartment of Immunology, Center for Cancer Immunology Research, University of Texas M. D. Anderson Cancer Center, Houston, TX 77030; bDepartment of Research and Development, Etablissement Français du Sang Rhône-Alpes Grenoble, 38701 La Tronche, France; and cDepartment of Biochemistry, Baylor College of Medicine, Houston, TX 77030 Edited by Ralph M. Steinman, The Rockefeller University, New York, NY, and approved July 14, 2010 (received for review May 10, 2010) Toll-like receptor 9 (TLR9) senses microbial DNA and triggers type I Microbial nucleic acids, including their genomic DNA/RNA IFN responses in plasmacytoid dendritic cells (pDCs). Previous and replicating intermediates, work as strong PAMPs (13), so studies suggest the presence of myeloid differentiation primary finding PRR-sensing pathogenic nucleic acids and investigating response gene 88 (MyD88)-dependent DNA sensors other than their signaling pathway is of general interest. Cytosolic RNA is TLR9 in pDCs. Using MS, we investigated C-phosphate-G (CpG)- recognized by RLRs, including RIG-I, melanoma differentiation- binding proteins from human pDCs, pDC-cell lines, and interferon associated gene 5 (MDA5), and laboratory of genetics and physi- regulatory factor 7 (IRF7)-expressing B-cell lines. CpG-A selectively ology 2 (LGP2). RIG-I senses 5′-triphosphate dsRNA and ssRNA bound the aspartate-glutamate-any amino acid-aspartate/histi- or short dsRNA with blunt ends. -

Composition of the Survival Motor Neuron (SMN) Complex in Drosophila Melanogaster
INVESTIGATION Composition of the Survival Motor Neuron (SMN) Complex in Drosophila melanogaster A. Gregory Matera,*,†,‡,§,**,1 Amanda C. Raimer,*,†,2 Casey A. Schmidt,*,† Jo A. Kelly,* Gaith N. Droby,*,† David Baillat,†† Sara ten Have,‡‡ Angus I. Lamond,‡‡ Eric J. Wagner,†† and Kelsey M. Gray*,†,2 *Integrative Program for Biological and Genome Sciences, †Curriculum in Genetics and Molecular Biology, ‡Lineberger § Comprehensive Cancer Center, Department of Biology, and **Department of Genetics, The University of North Carolina, Chapel Hill, NC 27599, ††Department of Biochemistry and Molecular Biology, University of Texas Medical Branch, Galveston, TX 77550, and ‡‡Centre for Gene Regulation and Expression, School of Life Sciences, University of Dundee, Dundee, DD15EH, UK ORCID IDs: 0000-0002-6406-0630 (A.G.M.); 0000-0003-0116-0340 (G.N.D.) ABSTRACT Spinal Muscular Atrophy (SMA) is caused by homozygous mutations in the human survival motor KEYWORDS neuron 1 (SMN1) gene. SMN protein has a well-characterized role in the biogenesis of small nuclear ribonucleo- locomotor proteins (snRNPs), core components of the spliceosome. SMN is part of an oligomeric complex with core binding function partners, collectively called Gemins. Biochemical and cell biological studies demonstrate that certain Gemins are ncRNA required for proper snRNP assembly and transport. However, the precise functions of most Gemins are unknown. proteomics To gain a deeper understanding of the SMN complex in the context of metazoan evolution, we investigated its RNP assembly composition in Drosophila melanogaster. Using transgenic flies that exclusively express Flag-tagged SMN from its SMN native promoter, we previously found that Gemin2, Gemin3, Gemin5, and all nine classical Sm proteins, including survival motor Lsm10 and Lsm11, co-purify with SMN. -
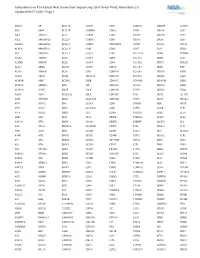
Comprehensive Pan-Cancer Next Generation Sequencing Solid Tumor Panel, Aberration List Updated 08-07-2020 --Page 1
Comprehensive Pan-Cancer Next Generation Sequencing Solid Tumor Panel, Aberration List Updated 08-07-2020 --Page 1 ABCC3 AR BCL11A CANT1 CDK1 CMKLR1 DAB2IP DUSP9 ABI1 ARAF BCL11B CAPRIN1 CDK12 CNBP DACH1 E2F1 ABL1 ARFRP1 BCL2 CAPZB CDK2 CNOT2 DACH2 E2F3 ABL2 ARHGAP20 BCL2A1 CARD11 CDK4 CNTN1 DAXX EBF1 ABLIM1 ARHGAP26 BCL2L1 CARM1 CDK5RAP2 CNTRL DCLK2 ECT2L ACACA ARHGEF12 BCL2L11 CARS CDK6 COG5 DCN EDIL3 ACE ARHGEF7 BCL2L2 CASC5 CDK7 COL11A1 DDB1 EDNRB ACER1 ARID1A BCL3 CASP3 CDK8 COL1A1 DDB2 EED ACSBG1 ARID1B BCL6 CASP7 CDK9 COL1A2 DDIT3 EEFSEC ACSL3 ARID2 BCL7A CASP8 CDKL5 COL3A1 DDR2 EGF ACSL6 ARID5B BCL9 CAV1 CDKN1A COL6A3 DDX10 EGFR ACVR1 ARIH2 BCOR CBFA2T3 CDKN1B COL9A3 DDX20 EGR1 ACVR1B ARNT BCORL1 CBFB CDKN1C COMMD1 DDX39B EGR2 ACVR1C ARRDC4 BCR CBL CDKN2A COX6C DDX3X EGR3 ACVR2A ASMTL BDNF CBLB CDKN2B CPNE1 DDX41 EGR4 ADD3 ASPH BHLHE22 CBLC CDKN2C CPS1 DDX5 EIF1AX ADM ASPSCR1 BICC1 CCDC28A CDKN2D CPSF6 DDX6 EIF4A2 AFF1 ASTN2 BIN1 CCDC6 CDX1 CRADD DEK EIF4E AFF3 ASXL1 BIRC3 CCDC88C CDX2 CREB1 DGKB ELF3 AFF4 ASXL2 BIRC6 CCK CEBPA CREB3L1 DGKI ELF4 AGR3 ATF1 BLM CCL2 CEBPB CREB3L2 DGKZ ELK4 AHCYL1 ATF3 BMP4 CCNA2 CEBPD CREBBP DICER1 ELL AHI1 ATG13 BMPR1A CCNB1IP1 CEBPE CRKL DIRAS3 ELN AHR ATG5 BRAF CCNB3 CENPF CRLF2 DIS3 ELOVL2 AHRR ATIC BRCA1 CCND1 CENPU CRTC1 DIS3L2 ELP2 AIP ATL1 BRCA2 CCND2 CEP170B CRTC3 DKK1 EML1 AK2 ATM BRCC3 CCND3 CEP57 CSF1 DKK2 EML4 AK5 ATP1B4 BRD1 CCNE1 CEP85L CSF1R DKK4 ENPP2 AKAP12 ATP8A2 BRD3 CCNG1 CHCHD7 CSF3 DLEC1 EP300 AKAP6 ATR BRD4 CCT6B CHD2 CSF3R DLL1 EP400 AKAP9 ATRNL1 BRIP1 CD19 CHD4 CSNK1A1 DLL3