A Study of Chemotherapy Resistance in Acute Myeloid Leukemia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human Lectins, Their Carbohydrate Affinities and Where to Find Them
biomolecules Review Human Lectins, Their Carbohydrate Affinities and Where to Review HumanFind Them Lectins, Their Carbohydrate Affinities and Where to FindCláudia ThemD. Raposo 1,*, André B. Canelas 2 and M. Teresa Barros 1 1, 2 1 Cláudia D. Raposo * , Andr1 é LAQVB. Canelas‐Requimte,and Department M. Teresa of Chemistry, Barros NOVA School of Science and Technology, Universidade NOVA de Lisboa, 2829‐516 Caparica, Portugal; [email protected] 12 GlanbiaLAQV-Requimte,‐AgriChemWhey, Department Lisheen of Chemistry, Mine, Killoran, NOVA Moyne, School E41 of ScienceR622 Co. and Tipperary, Technology, Ireland; canelas‐ [email protected] NOVA de Lisboa, 2829-516 Caparica, Portugal; [email protected] 2* Correspondence:Glanbia-AgriChemWhey, [email protected]; Lisheen Mine, Tel.: Killoran, +351‐212948550 Moyne, E41 R622 Tipperary, Ireland; [email protected] * Correspondence: [email protected]; Tel.: +351-212948550 Abstract: Lectins are a class of proteins responsible for several biological roles such as cell‐cell in‐ Abstract:teractions,Lectins signaling are pathways, a class of and proteins several responsible innate immune for several responses biological against roles pathogens. such as Since cell-cell lec‐ interactions,tins are able signalingto bind to pathways, carbohydrates, and several they can innate be a immuneviable target responses for targeted against drug pathogens. delivery Since sys‐ lectinstems. In are fact, able several to bind lectins to carbohydrates, were approved they by canFood be and a viable Drug targetAdministration for targeted for drugthat purpose. delivery systems.Information In fact, about several specific lectins carbohydrate were approved recognition by Food by andlectin Drug receptors Administration was gathered for that herein, purpose. plus Informationthe specific organs about specific where those carbohydrate lectins can recognition be found by within lectin the receptors human was body. -

Identification of Novel Genes in Human Airway Epithelial Cells Associated with Chronic Obstructive Pulmonary Disease (COPD) Usin
www.nature.com/scientificreports OPEN Identifcation of Novel Genes in Human Airway Epithelial Cells associated with Chronic Obstructive Received: 6 July 2018 Accepted: 7 October 2018 Pulmonary Disease (COPD) using Published: xx xx xxxx Machine-Based Learning Algorithms Shayan Mostafaei1, Anoshirvan Kazemnejad1, Sadegh Azimzadeh Jamalkandi2, Soroush Amirhashchi 3, Seamas C. Donnelly4,5, Michelle E. Armstrong4 & Mohammad Doroudian4 The aim of this project was to identify candidate novel therapeutic targets to facilitate the treatment of COPD using machine-based learning (ML) algorithms and penalized regression models. In this study, 59 healthy smokers, 53 healthy non-smokers and 21 COPD smokers (9 GOLD stage I and 12 GOLD stage II) were included (n = 133). 20,097 probes were generated from a small airway epithelium (SAE) microarray dataset obtained from these subjects previously. Subsequently, the association between gene expression levels and smoking and COPD, respectively, was assessed using: AdaBoost Classifcation Trees, Decision Tree, Gradient Boosting Machines, Naive Bayes, Neural Network, Random Forest, Support Vector Machine and adaptive LASSO, Elastic-Net, and Ridge logistic regression analyses. Using this methodology, we identifed 44 candidate genes, 27 of these genes had been previously been reported as important factors in the pathogenesis of COPD or regulation of lung function. Here, we also identifed 17 genes, which have not been previously identifed to be associated with the pathogenesis of COPD or the regulation of lung function. The most signifcantly regulated of these genes included: PRKAR2B, GAD1, LINC00930 and SLITRK6. These novel genes may provide the basis for the future development of novel therapeutics in COPD and its associated morbidities. -

Detailed Characterization of Human Induced Pluripotent Stem Cells Manufactured for Therapeutic Applications
Stem Cell Rev and Rep DOI 10.1007/s12015-016-9662-8 Detailed Characterization of Human Induced Pluripotent Stem Cells Manufactured for Therapeutic Applications Behnam Ahmadian Baghbaderani 1 & Adhikarla Syama2 & Renuka Sivapatham3 & Ying Pei4 & Odity Mukherjee2 & Thomas Fellner1 & Xianmin Zeng3,4 & Mahendra S. Rao5,6 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract We have recently described manufacturing of hu- help determine which set of tests will be most useful in mon- man induced pluripotent stem cells (iPSC) master cell banks itoring the cells and establishing criteria for discarding a line. (MCB) generated by a clinically compliant process using cord blood as a starting material (Baghbaderani et al. in Stem Cell Keywords Induced pluripotent stem cells . Embryonic stem Reports, 5(4), 647–659, 2015). In this manuscript, we de- cells . Manufacturing . cGMP . Consent . Markers scribe the detailed characterization of the two iPSC clones generated using this process, including whole genome se- quencing (WGS), microarray, and comparative genomic hy- Introduction bridization (aCGH) single nucleotide polymorphism (SNP) analysis. We compare their profiles with a proposed calibra- Induced pluripotent stem cells (iPSCs) are akin to embryonic tion material and with a reporter subclone and lines made by a stem cells (ESC) [2] in their developmental potential, but dif- similar process from different donors. We believe that iPSCs fer from ESC in the starting cell used and the requirement of a are likely to be used to make multiple clinical products. We set of proteins to induce pluripotency [3]. Although function- further believe that the lines used as input material will be used ally identical, iPSCs may differ from ESC in subtle ways, at different sites and, given their immortal status, will be used including in their epigenetic profile, exposure to the environ- for many years or even decades. -

Immune-Related Genes: Potential Regulators and Drug Therapeutic Targets in Hypertrophic Cardiomyopathy
Hindawi Journal of Nanomaterials Volume 2021, Article ID 5528347, 14 pages https://doi.org/10.1155/2021/5528347 Research Article Immune-Related Genes: Potential Regulators and Drug Therapeutic Targets in Hypertrophic Cardiomyopathy Wei Liu ,1 Ju Ye,2 Jinqiang Cai ,3 Feng Xie,4 Mengjie Tang,5 and QinJing Yang 1 1Department of Pharmacy, The Third Xiangya Hospital, Central South University, Changsha 410013, China 2Department of Pharmacy, Zunyi Medical University, Zunyi 563000, China 3Department of Pharmacy, Xiangya Medical College, Central South University, Changsha 410013, China 4Department of Pharmacy, The Nanshan District Maternity & Child Healthcare Hospital of Shenzhen, Shenzhen 518052, China 5Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha 410013, China Correspondence should be addressed to QinJing Yang; [email protected] Received 29 January 2021; Revised 5 February 2021; Accepted 9 February 2021; Published 3 March 2021 Academic Editor: Tingting Hong Copyright © 2021 Wei Liu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background. Accumulating evidence shows that the innate immune system is a key player in cardiovascular repair and regeneration, but little is known about the role of immune-related genes (IRGs) in hypertrophic cardiomyopathy (HCM). Methods. The differential mRNA expression profiles of HCM samples were downloaded from the Gene Expression Omnibus (GEO) dataset (GSE89714), and the IRG expression profile was obtained from the ImmPort database. The regulatory pathways of IRGs in HCM were screened out through discrepantly expressive genes (DEGs) analysis, enrichment of gene function/pathway analysis, and protein-protein interaction (PPI) network. -

Identification of a Clinically Relevant Androgen-Dependent Gene Signature in Prostate Cancer
Published OnlineFirst February 15, 2011; DOI: 10.1158/0008-5472.CAN-10-2512 Cancer Tumor and Stem Cell Biology Research Identification of a Clinically Relevant Androgen-Dependent Gene Signature in Prostate Cancer Hannelore V. Heemers1, Lucy J. Schmidt3, Zhifu Sun4, Kevin M. Regan3, S. Keith Anderson5, Kelly Duncan1, Dan Wang2, Song Liu2, Karla V. Ballman5, and Donald J. Tindall3 Abstract The androgen receptor (AR) is the principal target for treatment of non–organ-confined prostate cancer (PCa). Androgen deprivation therapies (ADT) directed against the AR ligand–binding domain do not fully inhibit androgen-dependent signaling critical for PCa progression. Thus, information that could direct the development of more effective ADTs is desired. Systems and bioinformatics approaches suggest that considerable variation exists in the mechanisms by which AR regulates expression of effector genes, pointing to a role for secondary transcription factors. A combination of microarray and in silico analyses led us to identify a 158-gene signature that relies on AR along with the transcription factor SRF (serum response factor), representing less than 6% of androgen-dependent genes. This AR-SRF signature is sufficient to distinguish microdissected benign and malignant prostate samples, and it correlates with the presence of aggressive disease and poor outcome. The AR- SRF signature described here associates more strongly with biochemical failure than other AR target gene signatures of similar size. Furthermore, it is enriched in malignant versus benign prostate tissues, compared with other signatures. To our knowledge, this profile represents the first demonstration of a distinct mechanism of androgen action with clinical relevance in PCa, offering a possible rationale to develop novel and more effective forms of ADT. -
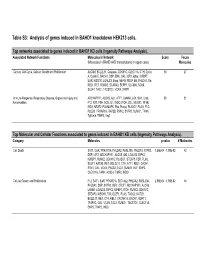
Table S3: Analysis of Genes Induced in BAHD1 Knockdown HEK213 Cells
Table S3: Analysis of genes induced in BAHD1 knockdown HEK213 cells. Top networks associated to genes induced in BAHD1 KD cells (Ingenuity Pathways Analysis). Associated Network Functions Molecules in Network Score Focus (Molecules in BAHD1-KD transcriptome in upper case) Molecules Cancer, Cell Cycle, Cellular Growth and Proliferation ALCAM, BCL2L11, Caspase, CDKN1C, CLEC11A, CTH, Cyclin 54 27 A, Cyclin E, DACH1, DSP, ERK, GAL, IGF2, Igfbp, IGFBP7, IL6R, KISS1R, LGALS3, Mmp, NEFM, PDGF BB, PHLDA1, Rb, RBL1, RET, RUNX2, SCARA3, SEPP1, SLC6A6, SOX8, SULF1, TAC1, TACSTD1, VCAN, WWP1 Immune Response, Respiratory Disease, Organismal Injury and ADCYAP1R1, ALOX5, Ap1, ATF1, CaMKII, COL13A1, Creb, 39 21 Abnormalities F12, F2R, FSH, hCG, IL1, INDO, ITCH, LDL, MEOX1, NFkB, NID1, NR2F2, P38 MAPK, Pka, Pkc(s), PLA2G7, PLAU, PLC, PLCD3, PRKAR1A, RAP2B, RIPK2, S1PR3, SUMO1, TANK, Tgf beta, TIMP2, Vegf Top Molecular and Cellular Functions associated to genes induced in BAHD1 KD cells (Ingenuity Pathways Analysis). Category Molecules p-value # Molecules Cell Death SAT1, IL6R, PRKAR1A, PHLDA2, RASL10A, PHLDA1, S1PR3, 1,56E-04 - 1,90E-02 43 DSP, IGF2, ADCYAP1R1, ALOX5, QKI, LGALS3, RIPK2, IGFBP7, RUNX2, CDKN1C, PLA2G7, STEAP3, F2R, PLAU, SULF1, KAT2B, RET, BCL2L11, CTH, ATF1, RBL1, DACH1, RTN1, GAL, VCAN, PACS2, TAC1, SUMO1, HLF, EMP2, CLEC11A, TANK, ACSL4, TIMP2, INDO Cellular Growth and Proliferation F12, SAT1, IL6R, PRKAR1A, SEC14L2, PHLDA2, RASL10A, 2,35E-04 - 1,90E-02 46 PHLDA1, DSP, S1PR3, IGF2, CRLF1, ADCYAP1R1, ALOX5, LAMB1, LGALS3, RIPK2, IGFBP7, -

Table S1. 103 Ferroptosis-Related Genes Retrieved from the Genecards
Table S1. 103 ferroptosis-related genes retrieved from the GeneCards. Gene Symbol Description Category GPX4 Glutathione Peroxidase 4 Protein Coding AIFM2 Apoptosis Inducing Factor Mitochondria Associated 2 Protein Coding TP53 Tumor Protein P53 Protein Coding ACSL4 Acyl-CoA Synthetase Long Chain Family Member 4 Protein Coding SLC7A11 Solute Carrier Family 7 Member 11 Protein Coding VDAC2 Voltage Dependent Anion Channel 2 Protein Coding VDAC3 Voltage Dependent Anion Channel 3 Protein Coding ATG5 Autophagy Related 5 Protein Coding ATG7 Autophagy Related 7 Protein Coding NCOA4 Nuclear Receptor Coactivator 4 Protein Coding HMOX1 Heme Oxygenase 1 Protein Coding SLC3A2 Solute Carrier Family 3 Member 2 Protein Coding ALOX15 Arachidonate 15-Lipoxygenase Protein Coding BECN1 Beclin 1 Protein Coding PRKAA1 Protein Kinase AMP-Activated Catalytic Subunit Alpha 1 Protein Coding SAT1 Spermidine/Spermine N1-Acetyltransferase 1 Protein Coding NF2 Neurofibromin 2 Protein Coding YAP1 Yes1 Associated Transcriptional Regulator Protein Coding FTH1 Ferritin Heavy Chain 1 Protein Coding TF Transferrin Protein Coding TFRC Transferrin Receptor Protein Coding FTL Ferritin Light Chain Protein Coding CYBB Cytochrome B-245 Beta Chain Protein Coding GSS Glutathione Synthetase Protein Coding CP Ceruloplasmin Protein Coding PRNP Prion Protein Protein Coding SLC11A2 Solute Carrier Family 11 Member 2 Protein Coding SLC40A1 Solute Carrier Family 40 Member 1 Protein Coding STEAP3 STEAP3 Metalloreductase Protein Coding ACSL1 Acyl-CoA Synthetase Long Chain Family Member 1 Protein -
Dissecting Human Skeletal Stem Cell Ontogeny by Single-Cell Transcriptomic
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.22.423948; this version posted December 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Dissecting human skeletal stem cell ontogeny by single-cell transcriptomic 2 and functional analyses 3 Jian He1,*, Jing Yan1,*, Jianfang Wang2,*, Liangyu Zhao3,*, Qian Xin1,*, Yang Zeng4, 4 Yuxi Sun5, Han Zhang6, Zhijie Bai1, Zongcheng Li4, Yanli Ni4, Yandong Gong1, 5 Yunqiao Li1, Han He1, Zhilei Bian7,8, Yu Lan7,8, Chunyu Ma9, Lihong Bian9, Heng 6 Zhu10, Bing Liu1,4,7,11 & Rui Yue2 7 1 State Key Laboratory of Proteomics, Academy of Military Medical Sciences, 8 Academy of Military Sciences, Beijing 100071, China 9 2 Institute for Regenerative Medicine, Shanghai East Hospital, Shanghai Key 10 Laboratory of Signaling and Disease Research, Frontier Science Center for Stem 11 Cell Research, School of Life Sciences and Technology, Tongji University, Shanghai 12 200092, China 13 3 Department of Orthopedics, Changzheng Hospital, Second Military Medical 14 University, Shanghai, 200003, China. 15 4 State Key Laboratory of Experimental Hematology, Fifth Medical Center of Chinese 16 PLA General Hospital, Beijing 100071, China 17 5 Department of Cardiology, Shanghai Tenth People's Hospital, Tongji University 18 School of Medicine, Shanghai, 200072, China 19 6 Department of Transfusion, Daping Hospital, Army Medical University, Chongqing, 20 400042, China 21 7 Key Laboratory for Regenerative Medicine of Ministry of Education, Institute of 22 Hematology, School of Medicine, Jinan University, Guangzhou 510632, China 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.12.22.423948; this version posted December 22, 2020. -
Single-Cell Transcriptome Analysis Reveals Mesenchymal Stem Cells In
bioRxiv preprint doi: https://doi.org/10.1101/2021.09.02.458742; this version posted September 3, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Single-cell transcriptome analysis reveals mesenchymal stem cells 2 in cavernous hemangioma 3 Fulong Ji1$, Yong Liu2$, Jinsong Shi3$, Chunxiang Liu1, Siqi Fu1 4 Heng Wang1, Bingbing Ren1, Dong Mi4, Shan Gao2*, Daqing Sun1* 5 1 Department of Paediatric Surgery, Tianjin Medical University General Hospital, Tianjin 300052, P.R. China. 6 China. 7 2 College of Life Sciences, Nankai University, Tianjin, Tianjin 300071, P.R. China; 8 3 National Clinical Research Center of Kidney Disease, Jinling Hospital, Nanjing University School of 9 Medicine, Nanjing, Jiangsu 210016, P.R. China; 10 4 School of Mathematical Sciences, Nankai University, Tianjin, Tianjin 300071, P.R. China; 11 12 13 $ These authors contributed equally to this paper. 14 * Corresponding authors. 15 SG:[email protected] 16 DS:[email protected] 17 bioRxiv preprint doi: https://doi.org/10.1101/2021.09.02.458742; this version posted September 3, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 18 Abstract 19 A cavernous hemangioma, well-known as vascular malformation, is present at birth, grows 20 proportionately with the child, and does not undergo regression. -

Gene Modules Associated with Human Diseases Revealed by Network
bioRxiv preprint doi: https://doi.org/10.1101/598151; this version posted June 15, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Gene modules associated with human diseases revealed by network analysis Shisong Ma1,2*, Jiazhen Gong1†, Wanzhu Zuo1†, Haiying Geng1, Yu Zhang1, Meng Wang1, Ershang Han1, Jing Peng1, Yuzhou Wang1, Yifan Wang1, Yanyan Chen1 1. Hefei National Laboratory for Physical Sciences at the Microscale, School of Life Sciences, University of Science and Technology of China, Hefei, Anhui 230027, China 2. School of Data Science, University of Science and Technology of China, Hefei, Anhui 230027, China * To whom correspondence should be addressed. Email: [email protected] † These authors contribute equally. 1 bioRxiv preprint doi: https://doi.org/10.1101/598151; this version posted June 15, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. ABSTRACT Despite many genes associated with human diseases have been identified, disease mechanisms often remain elusive due to the lack of understanding how disease genes are connected functionally at pathways level. Within biological networks, disease genes likely map to modules whose identification facilitates etiology studies but remains challenging. We describe a systematic approach to identify disease-associated gene modules. -

Biomedical Informatics
BIOMEDICAL INFORMATICS Abstract GENE LIST AUTOMATICALLY DERIVED FOR YOU (GLAD4U): DERIVING AND PRIORITIZING GENE LISTS FROM PUBMED LITERATURE JEROME JOURQUIN Thesis under the direction of Professor Bing Zhang Answering questions such as ―Which genes are related to breast cancer?‖ usually requires retrieving relevant publications through the PubMed search engine, reading these publications, and manually creating gene lists. This process is both time-consuming and prone to errors. We report GLAD4U (Gene List Automatically Derived For You), a novel, free web-based gene retrieval and prioritization tool. The quality of gene lists created by GLAD4U for three Gene Ontology terms and three disease terms was assessed using ―gold standard‖ lists curated in public databases. We also compared the performance of GLAD4U with that of another gene prioritization software, EBIMed. GLAD4U has a high overall recall. Although precision is generally low, its prioritization methods successfully rank truly relevant genes at the top of generated lists to facilitate efficient browsing. GLAD4U is simple to use, and its interface can be found at: http://bioinfo.vanderbilt.edu/glad4u. Approved ___________________________________________ Date _____________ GENE LIST AUTOMATICALLY DERIVED FOR YOU (GLAD4U): DERIVING AND PRIORITIZING GENE LISTS FROM PUBMED LITERATURE By Jérôme Jourquin Thesis Submitted to the Faculty of the Graduate School of Vanderbilt University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Biomedical Informatics May, 2010 Nashville, Tennessee Approved: Professor Bing Zhang Professor Hua Xu Professor Daniel R. Masys ACKNOWLEDGEMENTS I would like to express profound gratitude to my advisor, Dr. Bing Zhang, for his invaluable support, supervision and suggestions throughout this research work. -
DNA Methylation Signature of Human Hippocampus in Alzheimer's
Altuna et al. Clinical Epigenetics (2019) 11:91 https://doi.org/10.1186/s13148-019-0672-7 RESEARCH Open Access DNA methylation signature of human hippocampus in Alzheimer’s disease is linked to neurogenesis Miren Altuna1,2, Amaya Urdánoz-Casado1, Javier Sánchez-Ruiz de Gordoa1,2, María V. Zelaya3, Alberto Labarga4, Julie M. J. Lepesant5, Miren Roldán1, Idoia Blanco-Luquin1, Álvaro Perdones4, Rosa Larumbe1,2, Ivonne Jericó2, Carmen Echavarri1,2, Iván Méndez-López1,6, Luisa Di Stefano5 and Maite Mendioroz1,2* Abstract Background: Drawing the epigenome landscape of Alzheimer’s disease (AD) still remains a challenge. To characterize the epigenetic molecular basis of the human hippocampus in AD, we profiled genome-wide DNA methylation levels in hippocampal samples from a cohort of pure AD patients and controls by using the Illumina 450K methylation arrays. Results: Up to 118 AD-related differentially methylated positions (DMPs) were identified in the AD hippocampus, and extended mapping of specific regions was obtained by bisulfite cloning sequencing. AD-related DMPs were significantly correlated with phosphorylated tau burden. Functional analysis highlighted that AD-related DMPs were enriched in poised promoters that were not generally maintained in committed neural progenitor cells, as shown by ChiP-qPCR experiments. Interestingly, AD-related DMPs preferentially involved neurodevelopmental and neurogenesis- related genes. Finally, InterPro ontology analysis revealed enrichment in homeobox-containing transcription factors in the set of AD-related DMPs. Conclusions: These results suggest that altered DNA methylation in the AD hippocampus occurs at specific regulatory regions crucial for neural differentiation supporting the notion that adult hippocampal neurogenesis may play a role in AD through epigenetic mechanisms.