Planetary Protection Plan for Exomars
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Using a Nuclear Explosive Device for Planetary Defense Against an Incoming Asteroid
Georgetown University Law Center Scholarship @ GEORGETOWN LAW 2019 Exoatmospheric Plowshares: Using a Nuclear Explosive Device for Planetary Defense Against an Incoming Asteroid David A. Koplow Georgetown University Law Center, [email protected] This paper can be downloaded free of charge from: https://scholarship.law.georgetown.edu/facpub/2197 https://ssrn.com/abstract=3229382 UCLA Journal of International Law & Foreign Affairs, Spring 2019, Issue 1, 76. This open-access article is brought to you by the Georgetown Law Library. Posted with permission of the author. Follow this and additional works at: https://scholarship.law.georgetown.edu/facpub Part of the Air and Space Law Commons, International Law Commons, Law and Philosophy Commons, and the National Security Law Commons EXOATMOSPHERIC PLOWSHARES: USING A NUCLEAR EXPLOSIVE DEVICE FOR PLANETARY DEFENSE AGAINST AN INCOMING ASTEROID DavidA. Koplow* "They shall bear their swords into plowshares, and their spears into pruning hooks" Isaiah 2:4 ABSTRACT What should be done if we suddenly discover a large asteroid on a collision course with Earth? The consequences of an impact could be enormous-scientists believe thatsuch a strike 60 million years ago led to the extinction of the dinosaurs, and something ofsimilar magnitude could happen again. Although no such extraterrestrialthreat now looms on the horizon, astronomers concede that they cannot detect all the potentially hazardous * Professor of Law, Georgetown University Law Center. The author gratefully acknowledges the valuable comments from the following experts, colleagues and friends who reviewed prior drafts of this manuscript: Hope M. Babcock, Michael R. Cannon, Pierce Corden, Thomas Graham, Jr., Henry R. Hertzfeld, Edward M. -

View Conducted by Its Standing Review Board (SRB)
Science Committee Report Dr. Wes Huntress, Chair 1 Science Committee Members Wes Huntress, Chair Byron Tapley, (Vice Chair) University of Texas-Austin, Chair of Earth Science Alan Boss, Carnegie Institution, Chair of Astrophysics Ron Greeley, Arizona State University, Chair of Planetary Science Gene Levy, Rice University , Chair of Planetary Protection Roy Torbert, University of New Hampshire, Chair of Heliophysics Jack Burns, University of Colorado Noel Hinners, Independent Consultant *Judith Lean, Naval Research Laboratory Michael Turner, University of Chicago Charlie Kennel, Chair of Space Studies Board (ex officio member) * = resigned July 16, 2010 2 Agenda • Science Results • Programmatic Status • Findings & Recommendations 3 Unusual Thermosphere Collapse • Deep drop in Thermospheric (50 – 400 km) density • Deeper than expected from solar cycle & CO2 4 Aeronomy of Ice in the Mesosphere (AIM) unlocking the secrets of Noctilucent Clouds (NLCs) Form 50 miles above surface in polar summer vs ~ 6 miles for “norm79al” clouds. NLCs getting brighter; occurring more often. Why? Linked to global change? AIM NLC Image June 27, 2009 - AIM measured the relationship between cloud properties and temperature - Quantified for the first time, the dramatic response to small changes, 10 deg C, in temperature - T sensitivity critical for study of global change effects on mesosphere Response to Gulf Oil Spill UAVSAR 23 June 2010 MODIS 31 May 2010 ASTER 24 May 2010 Visible Visible/IR false color Satellite instruments: continually monitoring the extent of -

Planetary Protection Requirements for Human and Robotic Missions to Mars
Concepts and Approaches for Mars Exploration (2012) 4331.pdf PLANETARY PROTECTION REQUIREMENTS FOR HUMAN AND ROBOTIC MISSIONS TO MARS. R. Mogul1, P. D. Stabekis2, M. S. Race3, and C. A. Conley4, 1California State Polytechnic University, Pomona, 3801 W. Temple Ave, Pomona, CA 91768 ([email protected]), 2Genex Systems, 525 School St. SW, Suite 201, Washington D.C. 20224 ([email protected]), 3SETI Institute, Mountain View, CA 94043, 4NASA Head- quarters, Washington D.C. 20546 ([email protected]) Introduction: Ensuring the scientific integrity of mission. Hardware involved in the acquisition and Mars exploration, and protecting the Earth and the storage of samples from Mars must be designed to human population from potential biohazards, requires protect Mars material from Earth contamination, and the incorporation of planetary protection into human ensure appropriate cleanliness from before launch spaceflight missions as well as robotic precursors. All through return to Earth. Technologies needed to ensure missions returning samples from Mars are required to sample cleanliness at levels similar to those maintained comply with stringent planetary protection require- by the Viking project, in the context of modern space- ments for this purpose, recent refinements to which are craft materials, are yet to be developed. reported here. The objectives of planetary protection policy are As indicated in NASA Policy Directive NPD the same for both human and robotic missions; howev- 8020.7G (section 5c), to ensure compliance with the er, the specific implementation requirements will nec- Outer Space Treaty planetary protection is a mandatory essarily be different. Human missions will require component for all solar system exploration, including additional planetary protection approaches that mini- human missions. -

PLANETARY PROTECTION and REGULATING HUMAN HEALTH: a RISK THAT IS NOT ZERO Victoria Sutton1
(3) FINAL MACRO VERSION - VICTORIA SUTTON ARTICLE (PP. 71-102) (DO NOT DELETE) 3/9/2020 9:40 AM 19 Hous. J. Health L. & Policy 71 Copyright © 2019 Victoria Sutton Houston Journal of Health Law & Policy PLANETARY PROTECTION AND REGULATING HUMAN HEALTH: A RISK THAT IS NOT ZERO Victoria Sutton1 INTRODUCTION ............................................................................................ 73 I. LESSONS FROM HUMAN HISTORY ........................................................... 75 II. U.S. LEADERSHIP, INTERNATIONAL LAW, AND GUIDELINES FOR BACK CONTAMINATION—A SHORT HISTORY OF PLANETARY PROTECTION AND BIOCONTAINMENT ................................................... 79 A. International Law and Biocontainment Binding All Nations .......................................................................................... 82 B. Private Space Travel Must Comply with Planetary Protection ...................................................................................... 84 C. Enforceability is a Weakness ......................................................... 85 III. POLICY INDICATIONS THAT A RENEWED FOCUS IS NEEDED FOR BACK CONTAMINATION ........................................................................ 86 A. The Elimination of Human Health and Quarantine from U.S. Planetary Protection Protocols ........................................... 86 1 Victoria Sutton, MPA, PhD, JD, is the Paul Whitfield Horn Professor at Texas Tech University School of Law. Dr. Sutton is the former Assistant Director at the White House Science Office -

Biological Planetary Protection for Human Missions to Mars
NASA NID 8715.129 Interim Effective Date: July 9, 2020 Directive Expiration Date: July 9, 2021 Subject: Biological Planetary Protection for Human Missions to Mars Responsible Office: Office of Safety and Mission Assurance Table of Contents Preface P.1 Purpose P.2 Applicability P.3 Authority P.4 Applicable Documents P.5 Measurement/Verification P.6 Cancellation Chapter 1. Mitigating Backward and Forward Harmful Biological Contamination from Human Missions to Mars 1.1 Overview 1.2 Guidance for Biological Planetary Protection for Human Missions to Mars 1.3 NASA Policy on Biological Planetary Protection for Human Missions to Mars Appendix A. References 1 Preface P.1 Purpose a. This directive defines NASA’s obligation to avoid harmful forward and backward biological contamination under Article IX of the Treaty on Principles Governing the Activities of States in the Exploration and Use of Outer Space, including the Moon and Other Celestial Bodies (the "Outer Space Treaty"), October 19, 1967. b. This directive specifically addresses the control of forward biological contamination of Mars and backward biological contamination of the Earth-Moon system associated with human presence in space vehicles intended to land, orbit, flyby, and return from Mars. c. The 1967 Outer Space Treaty provides in relevant part: “States Parties to the Treaty shall pursue studies of outer space, including the Moon and other celestial bodies, and conduct exploration of them so as to avoid their harmful contamination and also adverse changes in the environment of the Earth resulting from the introduction of extraterrestrial matter and, where necessary, shall adopt appropriate measures for this purpose.” NASA recognizes that the 1967 Outer Space Treaty (OST) sets forth legal requirements on U.S. -

Planetary Protection Background & Motivation
Planetary Protection Background & Motivation Gerhard Kminek Independent Safety Office European Space Agency ESA UNCLASSIFIED - For Official Use What planetary protection is not Fireball exploded above Chelyabinsk city in the morning of 15 Feb. 2013 It is not about asteroid defense → Covered in the Near Earth Objects (NEO) and Space Situational Awareness (SSA) programs Credit: ESA It is not about space debris → Covered in the Space Surveillance and Tracking (SST), space debris, and sustainability programs Credit: NASA-JPL/MER It is not about cultural or natural world heritage → Covered by UNESCO based on a convention (for Earth) and the COSPAR Panel on Exploration (for space) Credit: Mars Daily It is not a green party for space ESA UNCLASSIFIED - For Official Use Goals for planetary protection Ensure that scientific investigations related to the origin and distribution of life are not compromised Picture credit: A. L. Hildebrand Protect our investment in space science & exploration Unique opportunity to learn more about the origin of life in a way that is no longer possible on Earth And than there is the more philosophical issue about the Drake equation Figure credit: Bada and Lazcano, Science 296, 2002 Protect the Earth from the potential hazard posed by extraterrestrial matter carried by a spacecraft returning from an interplanetary mission Simple prudence - protect the Earth! In line with the precautionary principle of environmental protection Bart Simpson, Dec. 17, 2000, “Skinner’s Sense of Snow” ESA UNCLASSIFIED - For Official Use History of planetary protection “…we are in the awkward situation of being able to spoil certain possibilities for scientific investigations for a considerable interval before we can constructively realize them…we urgently need to give some thought to the conservative measures needed to protect future scientific objectives on the moon and the planets…” J. -

Asteroid Retrieval Feasibility Study
Asteroid Retrieval Feasibility Study 2 April 2012 Prepared for the: Keck Institute for Space Studies California Institute of Technology Jet Propulsion Laboratory Pasadena, California 1 2 Authors and Study Participants NAME Organization E-Mail Signature John Brophy Co-Leader / NASA JPL / Caltech [email protected] Fred Culick Co-Leader / Caltech [email protected] Co -Leader / The Planetary Louis Friedman [email protected] Society Carlton Allen NASA JSC [email protected] David Baughman Naval Postgraduate School [email protected] NASA ARC/Carnegie Mellon Julie Bellerose [email protected] University Bruce Betts The Planetary Society [email protected] Mike Brown Caltech [email protected] Michael Busch UCLA [email protected] John Casani NASA JPL [email protected] Marcello Coradini ESA [email protected] John Dankanich NASA GRC [email protected] Paul Dimotakis Caltech [email protected] Harvard -Smithsonian Center for Martin Elvis [email protected] Astrophysics Ian Garrick-Bethel UCSC [email protected] Bob Gershman NASA JPL [email protected] Florida Institute for Human and Tom Jones [email protected] Machine Cognition Damon Landau NASA JPL [email protected] Chris Lewicki Arkyd Astronautics [email protected] John Lewis University of Arizona [email protected] Pedro Llanos USC [email protected] Mark Lupisella NASA GSFC [email protected] Dan Mazanek NASA LaRC [email protected] Prakhar Mehrotra Caltech [email protected] -

The Esa Exploration Programme – Exomars and Beyond
Lunar and Planetary Science XXXVI (2005) 2408.pdf THE ESA EXPLORATION PROGRAMME – EXOMARS AND BEYOND. G. Kminek1 and the Exploration Team, 1European Space Agency, D/HME, Keplerlaan 1, 2200 AG Noordwijk, The Netherlands, [email protected]. Management and Organization: The countries Technology Development for Exploration: Two participating in the European Exploration Programme categories for exploration technology developments Aurora have recently confirmed and increased their have been identified: contribution. The ESA Council has later approved the Generic Exploration Technology: They have a Agency’s budgets for 2005, including the budget for long-term strategic value, both for robotic and human Aurora. These developments enable major industrial exploration missions. Planetary protection, habitable activities to continue in line with original plans. These systems, risk assessment for human missions to planets, include work on the ExoMars mission and the Mars grey and black water recycling as well as psychological Sample Return mission, in-orbit assembly, rendezvous support for the Concordia Antarctic Station, Facility and docking, habitation and life support systems plus a for Integrated Planetary Exploration Simulations are broad range of technology development work. examples of selected generic exploration technologies. The Aurora Exploration Programme has been inte- Mission Specific Technologies: Specifically devel- grated into the Human Spaceflight and Microgravity oped for the programme’s missions, and will eventually Directorate , which now forms the Human Spaceflight, be implemented after reaching a minimum technology Microgravity, and Exploration Directorate of ESA. readiness level. EVD, sealing and sealing monitoring Early Robotic Missions: Robotic mission have technology, containment technology, specific instru- been identified as necessary prerequisite before send- ment developments, sample handling and distribution ing human to Mars. -

Advances in Planetary Protection at the Deep Space Gateway
Deep Space Gateway Science Workshop 2018 (LPI Contrib. No. 2063) 3111.pdf ADVANCES IN PLANETARY PROTECTION AT THE DEEP SPACE GATEWAY. J A Spry1, B Siegel2, M Race1, J D Rummel1, D E Pugel3, F J Groen2, G Kminek4, C A Conley5, N J Carosso3 (1SETI Research Inst, Mountain View CA (correspondence to [email protected]), 2NASA HQ, Washington DC, 3NASA GSFC, Greenbelt MD, 4ESA ESTEC, Noordwijk, Netherlands, 5NASA ARC, Mountain View CA). Introduction: Deep Space Gateway (DSG) envi- tary Protection Requirements for Human Missions is ronments provide an opportunity to address planetary part of that narrative, evaluating recent efforts, activi- protection knowledge gaps that will feed forward into ties and the state of the art in the context of current architecture concepts for surface operations at Mars COSPAR Planetary Protection Policy [2], collecting and for deep space travel, particularly concerning the inputs and identifying and prioritizing knowledge gaps return of astronauts to Earth after visiting the martian on the path to establishing numerical requirements surface. against which a crewed mission concept can be de- Spacefaring nations have, since the earliest years of signed and validated. the space programs and as reinforced by the Outer In this context, the DSG vehicle provides a plat- Space Treaty (OST) in 1967 [1], committed to protect- form for planetary protection investigations that could ing solar system objects from harmful contamination be performed to inform the planetary protection re- carried by interplanetary spacecraft. One result has quirements development process. This includes exper- been that the scientific investigation of extraterrestrial iments performed on the inside of the vehicle, to im- targets can be performed without the threat of being prove understanding of interactions between microbes compromised by terrestrial biological contaminants. -

Planetary Defense: Near-Earth Objects, Nuclear Weapons, and International Law James A
Hastings International and Comparative Law Review Volume 42 Article 2 Number 1 Winter 2019 Winter 2019 Planetary Defense: Near-Earth Objects, Nuclear Weapons, and International Law James A. Green Follow this and additional works at: https://repository.uchastings.edu/ hastings_international_comparative_law_review Part of the Comparative and Foreign Law Commons, and the International Law Commons Recommended Citation James A. Green, Planetary Defense: Near-Earth Objects, Nuclear Weapons, and International Law, 42 Hastings Int'l & Comp.L. Rev. 1 (2019). Available at: https://repository.uchastings.edu/hastings_international_comparative_law_review/vol42/iss1/2 This Article is brought to you for free and open access by the Law Journals at UC Hastings Scholarship Repository. It has been accepted for inclusion in Hastings International and Comparative Law Review by an authorized editor of UC Hastings Scholarship Repository. Planetary Defense: Near-Earth Objects, Nuclear Weapons, and International Law BY JAMES A. GREEN ABSTRACT The risk of a large Near-Earth Object (NEO), such as an asteroid, colliding with the Earth is low, but the consequences of that risk manifesting could be catastrophic. Recent years have witnessed an unprecedented increase in global political will in relation to NEO preparedness, following the meteoroid impact in Chelyabinsk, Russia in 2013. There also has been an increased focus amongst states on the possibility of using nuclear detonation to divert or destroy a collision- course NEO—something that a majority of scientific opinion now appears to view as representing humanity’s best, or perhaps only, option in extreme cases. Concurrently, recent developments in nuclear disarmament and the de-militarization of space directly contradict the proposed “nuclear option” for planetary defense. -

Planetary Protection and Article IX Of
Planetary Protection: Policies and Practices Tutorial Session 2 Planetary Protection Policy and Requirements G. Kminek and C. A. Conley June 2015 1 What planetary protection is not Fireball exploded above Chelyabinsk city in the It is not about asteroid/planetary defense morning of 15 Feb. 2013 Covered under Near Earth Objects and Space Situational Awareness Programs It is not about space debris Space Debris Image: ESA Covered by UN-COPUOS space debris mitigation guidelines International coordination through the Inter- Agency Space Debris Coordination Committee (IADC) MER on the surface of It is not about cultural or natural world Mars: NASA heritage Covered by UNESCO based on a convention (for Earth) and the COSPAR Panel on Exploration (for space) Credit: Mars Daily It is not a green party for space It is not about playing around with guns and ET Credit: Sony Pictures 2 Scope of Planetary Protection Ensure that scientific investigations related to the origin and distribution of life are not compromised Enabling capability Protect our investment in space science & exploration Protect the Earth from the potential hazard posed by extraterrestrial matter carried by a spacecraft returning from an interplanetary mission Bart Simpson, Dec. 17, 2000, “Skinner’s Simple prudence - protect the Earth! Sense of Snow” In line with the precautionary principle of environmental protection 3 History of Planetary Protection “…we are in the awkward situation of being able to spoil certain possibilities for scientific investigations for a considerable interval before we can constructively realize them…we urgently need to give some thought to the conservative measures needed to protect future scientific objectives on the moon and the planets…” J. -
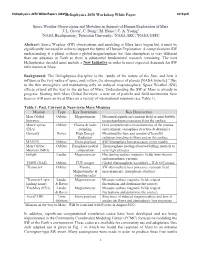
Heliophysics 2050 Workshop White Paper 1 Space Weather
Heliophysics 2050 White Papers (2021Heliophysics) 2050 Workshop White Paper 4010.pdf Space Weather Observations and Modeling in Support of Human Exploration of Mars J. L. Green1, C. Dong2, M. Hesse3, C. A. Young4 1NASA Headquarters; 2Princeton University; 3NASA ARC; 4NASA GSFC Abstract: Space Weather (SW) observations and modeling at Mars have begun but it must be significantly increased in order to support the future of Human Exploration. A comprehensive SW understanding at a planet without a global magnetosphere but thin atmosphere is very different than our situation at Earth so there is substantial fundamental research remaining. The next Heliophysics decadal must include a New Initiative in order to meet expected demands for SW information at Mars. Background: The Heliophysics discipline is the “study of the nature of the Sun, and how it influences the very nature of space and, in turn, the atmospheres of planets [NASA website].” Due to the thin atmosphere and maintaining only an induced magnetosphere, Space Weather (SW) effects extend all the way to the surface of Mars. Understanding the SW at Mars is already in progress. Starting with Mars Global Surveyor, a new set of particle and field instruments have been or will soon arrive at Mars on a variety of international missions (see Table 1). Table 1: Past, Current & Near-term Mars Missions Mission Type Key Instrument Key Discoveries Mars Global Orbiter Magnetometer Measured significant remnant field or mini-bubble Surveyor magnetospheres emanating from the surface Mars Express Orbiter