Quantification of the Degree of Substitution As a Diagnostic Tool for Assessing the Conservation Condition of Cellulose Nitrate and Acetate in Heritage Collections
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cellulose Acetate Microfilm Forum
Addressing Cellulose Acetate Microfilm from a British Library Perspective The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Shenton, Helen. 2005. Addressing cellulose acetate microfilm from a British library perspective. Liber Quarterly 15(2). Published Version http://liber.library.uu.nl/publish/articles/000134/article.pdf Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:3965108 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Addressing Cellulose Acetate Microfilm from a British Library perspective by HELEN SHENTON INTRODUCTION This paper is about cellulose acetate microfilm from the British Library perspective. It traces how acetate microfilm became an issue for the British Library and describes cellulose acetate deterioration. This is followed by details of what has already been done about the situation and what action is planned for the future. THE PROBLEM: CELLULOSE ACETATE DETERIORATION In order to tackle the issue it was important to be clear about what cellulose acetate deterioration is, and what can be done about it. Cellulose acetate replaced cellulose nitrate in the late 1940s and was called safety film. This was in use until the mid-1980s, when it was replaced by polyester. One definition of cellulose acetate film from the Image Permanence Institute’s Storage Guide for Acetate Film has not been bettered: “Cellulose acetate film is a modified form of cellulose, and can slowly decompose under the influence of heat, moisture and acids. -

General Introduction Sustainability Issues in the Preservation of Black and White Cellulose Esters Film- Based Negatives Collections
Élia Catarina Tavares Costa Roldão Licenciada em Conservação e Restauro A contribution for the preservation of cellulose esters black and white negatives Dissertação para obtenção do Grau de Doutor em Ciências da Conservação do Património, Especialidade em Ciências da Conservação Orientador: Doutora Ana Maria Martelo Ramos, Professora Associada, FCT NOVA Co-orientadores: Doutor Bertrand Lavédrine, CRC Doutor António Jorge D. Parola, Professor Associado com Agregação, FCT NOVA Júri: Presidente: Doutora Maria João Seixas de Melo, Professora Catedrática, FCTNOVA Arguentes: Doutor Hugh Douglas Burrows, Professor Catedrático Jubilado, FCT-UC Doutora Ana Isabel S. C. Delgado Martins, Directora do AHU-DGLAB Vogais: Doutora Ana Maria Martelo Ramos, Professora Associada, FCT NOVA Doutor João Pedro Martins de Almeida Lopes, Professor Auxiliar, FF- UL Novembro, 2018 A contribution for the preservation of cellulose esters black and white negatives Copyright © Élia Catarina Tavares Costa Roldão, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa. A Faculdade de Ciências e Tecnologia e Universidade Nova de Lisboa têm o direito, perpétuo e sem limites geográficos, de arquivar e publicar esta dissertação através de exemplares impressos reproduzidos em papel ou de forma digital, ou por qualquer outro meio conhecido ou que venha a ser inventado, e de divulgar através de repositórios científicos e de admitir a sua cópia e distribuição com objectivos educacionais ou de investigação, não comerciais, desde que seja dado crédito ao autor e editor. -

Object - Photo Report
Object - Photo Report 82.57.5 Name Moving image Photographer Kingery, Hugh Title Skiing in Colorado Non-original Title Skiing in Colorado Description Black-and-white 16mm movie on cellulose acetate film stock made circa 1950-1955, shot by Hugh Kingery. The film has not been reviewed (as of 7/16/2015) but appears to depict skiing in Colorado. The film is approximately 200 ft. in length. This film is #1 in a series of 3. Inscription The film reel in in a cardboard box with a Colorado Historical Society label. It reads: "Skiing in Colorado / 16mm film by Mr. Kingery / Gift of Mrs. Elinor Kingery / 4629 East Cedar Denver, Colorado 80222 / June 1982" ; Handwritten on label: "1982 prints" Subject Skis and skiing--Colorado, Amateur films Provenance Gift of Elinor Kingery, 1982. Collection Name Hugh M. and Elinor Kingery collection (Ph.00244) 82.57.6 Name Moving image Photographer Kingery, Hugh Title Skiing in Colorado Non-original Title Skiing in Colorado Description Black-and-white 16mm home movie on cellulose acetate film stock made circa 1950-1955, shot by Hugh Kingery. The film has not been reviewed (as of 7/16/2015) but appears to depict skiing in Colorado. The film is approximately 100 ft. in length. This film is #2 in a series of 3. Inscription The film reel in in a cardboard box with a Colorado Historical Society label. It reads: "Skiing in Colorado / 16mm film by Mr. Kingery / Gift of Mrs. Elinor Kingery / 4629 East Cedar Denver, Colorado 80222 / June 1982" ; Handwritten on label: "1982 prints" Subject Skis and skiing--Colorado Provenance Gift of Elinor Kingery, 1982. -

Mellon Guide.Qxd
THE FILM PRESERVATION GUIDE THE BASICS FOR ARCHIVES, LIBRARIES, AND MUSEUMS National Film Preservation Foundation San Francisco, California National Film Preservation Foundation 870 Market Street, Suite 1113 San Francisco, CA 94102 © 2004 by the National Film Preservation Foundation Library of Congress Cataloging-in-Publication Data The film preservation guide : the basics for archives, libraries, and museums. p. cm. Includes bibliographical references and index. ISBN 0-9747099-0-5 (alk. paper) 1. Motion picture film—Preservation. I. National Film Preservation Foundation (U.S.) TR886.3F58 2003 778.5’8—dc22 2003024032 CIP This publication was made possible through a grant from The Andrew W. Mellon Foundation. It may be downloaded as a PDF file from the National Film Preservation Foundation Web site: www.filmpreservation.org. Credits Except as noted below, all photographs were provided by Barbara Galasso and the L. Jeffrey Selznick School of Film Preservation at George Eastman House. The following contributed illustrations and text material: American Museum of Natural History (94), Anonymous (67), California Pacific Medical Center (57), Chace Productions Inc. (12 center and right), Duke University (48 top), Estate of Edith Lutyens Bel Geddes and the Harry Ransom Humanities Research Center at the University of Texas at Austin (84), Florida Moving Image Archive (91), Image Permanence Institute at the Rochester Institute of Technology (10 top), Library of Congress (48 bottom, 51, 63, 87), Minnesota Historical Society (92), National Center for Jewish Film (90), Nebraska State Historical Society (69, 73, 74), Northeast Historic Film (back cover, 62 bottom, 76, 85), Oklahoma Historical Society (5), Pacific Film Archive at the University of California at Berkeley (back cover), Sabucat Productions (93), UCLA Film and Tele- vision Archive (86), University of Alaska Fairbanks (40), University of South Carolina Newsfilm Library (89), Visual Communications (58). -

Rapid Identification of Cellulose Nitrate and Cellulose Acetate Film In
Carter et al. Herit Sci (2020) 8:51 https://doi.org/10.1186/s40494-020-00395-y RESEARCH ARTICLE Open Access Rapid identifcation of cellulose nitrate and cellulose acetate flm in historic photograph collections Elizabeth A. Carter1,2* , Brad Swarbrick2, Thérèse M. Harrison1 and Lucilla Ronai3 Abstract Cellulose nitrate transparent plastic flm was used by photographers and movie flmmakers from its release in the 1880s to the 1950s. The storage of this material is a challenge for cultural institutions because of its instability and haz- ardous nature, as nitrate is highly fammable and deteriorates over time. Historically, cellulose acetate gradually began to replace cellulose nitrate as it is not fammable. Despite its non-fammable properties, leading to cellulose acetate being called ‘safety’ flm, over time it became clear that it also deteriorates in hazardous ways. Identifcation of cellu- lose nitrate and cellulose acetate in collections is necessary for preservation and risk management to collections and humans. Both cellulose nitrate and cellulose acetate are found in gallery, library, archive and museum photographic collections. As a result, identifcation and management of this material might be the responsibility of curators, librar- ians, archivists, collection managers, registrars or conservators, depending on the institution stafng and structure. Currently, there is no single identifcation method readily available to heritage institutions that meets the require- ments to identify and distinguish between cellulose nitrate and cellulose acetate reliably, non-destructively and rapidly. In this study samples from both the Sydney University Museums and Australian National Maritime Museum collections have been analysed using infrared total refectance spectroscopy, a novel approach which demonstrates great potential for rapid and non-invasive identifcation particularly when combined with multivariate statistical analysis methods. -

The Acetate Negative Survey
THE ACETATE NEGATIVE SURVEY FINAL REPORT A PROJECT FUNDED BY THE UNIVERSITY OF LOUISVILLE AND THE NATIONAL MUSEUM ACT David G. Horvath University of Louisville Ekstrom Library Photographic Archives February 1987 The University of Louisville Photographic Archives Ekstrom Library Louisville, KY 40292 ACKNOWLEDGMENTS The subject of this study should be of great interest to those who are responsible for the preservation of historical photographic negatives. It has involved the cooperation and advice of many people who, piece by piece, have helped construct the method, process, and format of the final product. My wife, Mary has been very supportive and kept me from becoming discouraged by the endless details of the project. My colleagues at the Photographic Archives were most helpful in their constant encouragement, suggestions and warm friendship, especially Andy Anderson, Bill Carner, and Barbara Crawford; Michel C. Atlas was invaluable for her editorial contributions. I thank the National Museum Act of the Smithsonian Institution and the University of Louisville for their assistance and support, especially Barbara Schneider and Martha Bowman, the University of Louisville Librarian. I am indebted to all the institutions which allowed me access to their collections and provided me with a wealth of background information upon which much of this study is based. I owe special thanks to Ford Peatross, Carol Johnson, and Stephen Ostrow of the Library of Congress; Amy Doherty of the Special Collection Department at Syracuse University; Tim Peterson -

Preserving Your Collection of Film-Based Photographic Negatives
Preserving Your Collection of Film-Based Photographic Negatives Paul Messier Conservator of Photographic Materials and Works of Art on Paper Rocky Mountain Conservation Center Technical bulletin, funded by the Bay Foundation, 1993 TABLE OF CONTENTS ABSTRACT .................................................................................................................... 1 HISTORY OF FILM-BASED PHOTOGRAPHIC NEGATIVES.................................. 1 DETERIORATION OF FILM-BASED NEGATIVES................................................... 2 Deterioration of Nitrate................................................................................................ 2 Deteriorartion of diacetate and Triacetate.................................................................... 3 HAZARDS ...................................................................................................................... 3 Heath risks from deteriorated negatives....................................................................... 3 Fire hazard posed by nitrate negatives......................................................................... 4 IDENTIFICATION OF FILM-BASED NEGATIVES................................................... 4 STORAGE TECHNIQUES, MATERIALS, AND ENVIRONMENT........................... 4 General Recommendations .......................................................................................... 4 Storage environment .................................................................................................... 5 Cold storage -

Microfilm “Diseases”
Microfilm “Diseases” Common Microfilm diseases and how to prevent them Introduction • Most collecting institutions still hold materials in microformats. While the most common are the familiar 16mm and 35mm roll microfilm and the flat, card-shaped microfiche, microforms come in a number of shapes, sizes, film bases, and film types. Other, less commonly found microformats include aperture cards (a paper-stock card with a single microfilm frame, usually 35mm, often used for archiving engineering drawings) and microcards (similar to fiche but printed on photographic paper rather than film). Brief History • The concept of micro-photography was introduced in 1839 and was proposed as a document preservation technique in 1851. However, it was not until the era of flexible film that the idea caught on. Originally developed in the 1920s for use in banking, microfilm was embraced by libraries and other institutions: the Library of Congress began a major filming project in 1927. The era of newspaper filming began in 1935 when The New York Times started filming and publishing its print run. In the wake of these two considerable projects, cultural heritage organizations realized they could affordably film their collections not just for preservation, but also for access and distribution, much as digitization is used today. Film Bases Through the years, microforms have been manufactured on various film bases, including cellulose nitrate, cellulose acetate, and polyester. • Cellulose nitrate-based microforms, like other cellulose nitrate films, are highly flammable, prone to releasing hazardous gases over time, and subject to natural decomposition. By the early 1950s, commercial production of all formats of cellulose nitrate film had permanently ceased. -

María Fernanda Valverde Advanced Residency Program in Photograph Conservation Contents Preface
María Fernanda Valverde Advanced Residency Program in Photograph Conservation CONTENTS Preface ............................................................................................................................................. 3 Introduction .................................................................................................................................... 4 Paper Negatives ............................................................................................................................... 5 Collodion Glass Plate Negatives ...................................................................................................... 9 Gelatin Dry Plate Negatives ........................................................................................................... 14 Cellulose Nitrate Film Negatives .................................................................................................... 19 Cellulose Acetate Film Negatives................................................................................................... 24 Polyester Film Negatives ................................................................................................................ 29 General Bibliography ..................................................................................................................... 32 Glossary ........................................................................................................................................ 33 Endnotes ...................................................................................................................................... -
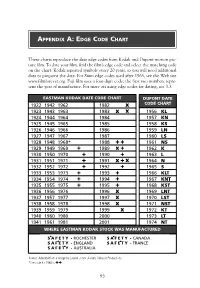
Eastman Kodak Date Code Chart
APPENDIX A: EDGE CODE CHART These charts reproduce the date edge codes from Kodak and Dupont motion pic- ture film. To date your film, find the film’s edge code and select the matching code on the chart. Kodak repeated symbols every 20 years, so you will need additional data to pinpoint the date. For 8mm edge codes used after 1965, see the Web site www.filmforever.org. Fuji film uses a four-digit code; the first two numbers repre- sent the year of manufacture. For more on using edge codes for dating, see 3.3. EASTMAN KODAK DATE CODE CHART DUPONT DATE 1922 1942 1962 ● ■ 1982 ● ■ x CODE CHART 1923 1943 1963 ● ▲ 1983 x ▲x 1956 KL 1924 1944 1964 ▲ ■ 1984 ▲■ ▲ 1957 KN 1925 1945 1965 ■ ● 1985 ■ ● ▲ 1958 KS 1926 1946 1966 ▲ ● 1986 ▲● ▲ 1959 LN 1927 1947 1967 ■ ▲ 1987 ■ ▲▲ 1960 LS 1928 1948 1968* ● ● ● 1988 + + ▲ 1961 NS 1929 1949 1969 + 1989 x + ▲ 1962 K 1930 1950 1970 ▲+ 1990 ▲+ ▲ 1963 L 1931 1951 1971 ● + 1991 x + x 1964 N 1932 1952 1972 ■ + 1992 ■ + ▲ 1965 S 1933 1953 1973 + ▲ 1993 + ▲▲ 1966 KLT 1934 1954 1974 + ● 1994 + ● ▲ 1967 KNT 1935 1955 1975 + ■ 1995 + ■ ▲ 1968 KST 1936 1956 1976 ● 1996 x ● ▲ 1969 LNT 1937 1957 1977 ■ 1997 x ■ ▲ 1970 LST 1938 1958 1978 ▲ 1998 x ▲▲ 1971 NST 1939 1959 1979 ● ● 1999 ● x ▲ 1972 KT 1940 1960 1980 ■ ■ 2000 ■ ■ ▲ 1973 LT 1941 1961 1981 ▲ ▲ 2001 ▲▲● 1974 NT WHERE EASTMAN KODAK STOCK WAS MANUFACTURED SAFETY• - ROCHESTER SAFETY• - CANADA SAFETY• - ENGLAND SAFETY• - FRANCE SAFETY• - AUSTRALIA Source: Adapted from a design by Lauren Jones-Joseph, Sabucat Productions. -

31St MAY 2019 LISBON, PORTUGAL
29th – 31st MAY 2019 LISBON, PORTUGAL 0 Title The Plastics Heritage Congress 2019: History, Limits and Possibilities Editors Inês Soares Maria Elvira Callapez Design Inês Soares Paulo Fuentez Printing Gráfica Digital ARP Date 29th – 31st May 2019, Lisbon, Portugal TABLE OF CONTENTS WELCOME TO THE PLASTICS HERITAGE CONGRESS 2019: HISTORY, LIMITS AND POSSIBILITIES .............................. 3 The congress ................................................................................................................................................................................... 4 About CIUHCT .................................................................................................................................................................................. 4 About PHEA (Plastics Heritage European Association) ...................................................................................................... 4 THE CONGRESS VENUE: MUSEU NACIONAL DOS COCHES/ NATIONAL COACH MUSEUM .............................................6 Library ................................................................................................................................................................................................ 7 Auditorium ........................................................................................................................................................................................ 7 Exhibition building......................................................................................................................................................................... -

University of Wisconsin, Investigation of Cellulose Nitrate Motion Picture
Narrative Section of a Successful Application The attached document contains the grant narrative of a previously funded grant application. It is not intended to serve as a model, but to give you a sense of how a successful application may be crafted. Every successful application is different, and each applicant is urged to prepare a proposal that reflects its unique project and aspirations. Prospective applicants should consult the NEH Division of Preservation and Access application guidelines at http://www.neh.gov/divisions/preservation for instructions. Applicants are also strongly encouraged to consult with the NEH Division of Preservation and Access staff well before a grant deadline. Note: The attachment only contains the grant narrative, not the entire funded application. In addition, certain portions may have been redacted to protect the privacy interests of an individual and/or to protect confidential commercial and financial information and/or to protect copyrighted materials. Project Title: Investigation of Cellulose Nitrate Motion Picture Film Chemical Decomposition & Association Fire Risk Institution: University of Wisconsin, Madison Project Director: Vance L. Kepley Grant Program: Research and Development 400 7th Street, SW, Floor 4, Washington, D.C. 20506 P 202.606.8570 F 202.606.8639 E [email protected] www.neh.gov Significance When it comes to nitrate film, our cultural heritage is inextricably linked to issues of human safety. Cellulose nitrate was used as the base for professional motion picture film for the first half of the 20th century, which means that virtually all works of commercial cinema made between the 1890s and the early 1950s were originally shot on nitrate stock.