Dr. Mark Butler
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -
Lobsters LOBSTERS§
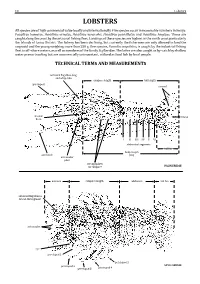
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

Taxonomy, Biology and Distribution of Lobsters
Taxonomy, Biology and Distribution of Lobsters 15 Rekha Devi Chakraborty and E.V.Radhakrishnan Crustacean Fisheries Division, Central Marine Fisheries Research Institute, Kochi-682 018 Lobsters are among the most prized of fisheries resources and of significant commercial interest in many countries. Because of their high value and esteemed culinary worth, much attention has been paid to lobsters in biological, fisheries, and systematic literature. They have a great demand in the domestic market as a delicacy and is a foreign exchange earner for the country. Taxonomic status Phylum: Arthropoda Subphylum: Crustacea Class: Malacostraca Subclass: Eumalacostraca Superorder: Eucarida Order: Decapoda Suborder: Macrura Reptantia The suborder Macrura Reptantia consists of three infraorders: Astacidea (Marine lobsters and freshwater crayfishes), Palinuridea (Spiny lobsters and slipper lobsters) and Thalassinidea (mud lobsters). The infraorder Astacidea Summer School on Recent Advances in Marine Biodiversity Conservation and Management 100 Rekha Devi Chakraborty and E.V.Radhakrishnan contains three superfamilies of which only one (the Infraorder Palinuridea, Superfamily Eryonoidea, Family Nephropoidea) is considered here. The remaining two Polychelidae superfamilies (Astacoidea and parastacoidea) contain the 1b. Third pereiopod never with a true chela,in most groups freshwater crayfishes. The superfamily Nephropoidea (40 chelae also absent from first and second pereiopods species) consists almost entirely of commercial or potentially 3a Antennal flagellum reduced to a single broad and flat commercial species. segment, similar to the other antennal segments ..... Infraorder Palinuridea, Superfamily Palinuroidea, The infraorder Palinuridea also contains three superfamilies Family Scyllaridae (Eryonoidea, Glypheoidea and Palinuroidea) all of which are 3b Antennal flagellum long, multi-articulate, flexible, whip- marine. The Eryonoidea are deepwater species of insignificant like, or more rigid commercial interest. -

Growth of the Spiny Lobster, Panulirus Homarus (Linnaeus), in Captivity
GROWTH OF THE SPINY LOBSTER, PANULIRUS HOMARUS (LINNAEUS), IN CAPTIVITY M. M. THOMAS' Central Marine Fisheries Research Institute; Regional Centre, Mandapam Camp ABSTRACT The growth of Panulirus homarus (Linnaeus) in captivity is traced in relation to moulting. The growth per moult of 4 to 9 mm carapace length and annual rate of growth of 30 mm in male and 17 mm in female are found to be in agreement wiih those of its congeners. Instances of moulting without growth and death during exuviation are also reported. INTRODUCTION Investigations on the growth of spiny lobsters have been undertaken by various workers. Moulting and subsequent increase in length and weight were traced by Kinoshita (1933) and Nakamura (1940) in Panulirus japonicus (Von Siebold). Lindberg (1955) calculated the increase in length of tagged animals, and Bakus (1960) estimated the yearly growth of females and males of Panulirus interruptus (Randall). Growth studies were undertaken by Simp son (1961) in Homarus vulgaris (Milne Edwards), Marshall (1948) and Dawson and Idyll (1951) in Panulirus argus (Latreille). Travis (1954) made a det ailed study of the moulting and consequent increase in size and weight in P. argus. The growth of the Indian spiny lobster, P. homarus has been estimated from the length frequency distribution by George (1967) while Mohamed and George (1971) have reported the actual increase in length observed during the mark-recovery experiments on the same species. MATERIAL AND METHODS Specimens of Panulirus homarus ranging in carapace length from 33 to 51 mm (total length 90 to 145 mm) were collected from the shore- seine landings from the Gulf of Mannar near Mandapam Camp and kept in glass aquaria with running sea water and provided with den-like shelters made of rocks and asbestos pieces. -

Marine Research and Management
Marine Research and Management Editors V.N. Pillai and N.G. Menon Central Marine Fisheries Research Institute (Indian Council of Agricultural Research) Tatapuram P.O., Cochin-682 014 Kerala, India 2000 50 Problems and prospects for lobster farming in ^^^^ India E.V. Radhakrishnan and M. Vijayakumaran ABSTRACT The potential for increastng the lobster yield from the wild is limited, as most of the stocks have reached optimum levels of produc tion. Hence attempts were made by the CMFR Institute to successfully grow the non-cannibalistic species under captivity in view of the above and demand for live lobster in export market. The paper reviews the previous culture attempts and the present status on the spiny lobster, distribution ofcuUurable species for farming andfattening, brood stock maintenance, larval culture, availability and growth ofpueruli, collec tion and transportation of Juvenile lobsters, fattening of subadults, growth acceleration by eye stalk ablation, farming in intertidal pits, environmental conditions for Juvenile rearing, stock density, food.diease, economics of farming andfattening, and the future pros pects in Indian condition. Introduction Lobsters are a low volume, high value resource which support one of the most valuable coastal fisheries in India. Annual landings increased from 350 t in 1965 to 4,100 t in 1985, but has generally declined since then. The current production is around 2,000 t, most being exported alive and in whole cooked form to the southeast Asian market. The potential for increasing the yield from the wild is limited, as most of the stocks are fully exploited and near their long term equilibrium yield. -

Layout-Winter School-2008-09.Pmd
CMFRI Course Manual Winter School on Recent Advances in Breeding and Larviculture of Marine Finfish and Shellfish 30.12.2008 -19.1.2009 Compiled and Edited by Dr. K. Madhu, Senior Scientist and Director, Winter school & Dr. Rema Madhu, Senior Scientist and Co-ordinator Central Marine Fisheries Research Institute Central Marine Fisheries Research Institute (Indian Council of Agricultural Research) P.B.No.1603, Marine Drive North Extension, Ernakulam North ,P.O. Cochin, KERALA – INDIA - 682018 OVERVIEW OF LOBSTER FARMING E.V. Radhakrishnan, Principal Scientist & Head CFD, Central Marine Fisheries Research Institute, Kochi- 682018. E mail: [email protected] Introduction Lobsters are high valued seafood with well established export markets around the world and form extremely important fisheries in many countries. World’s production of palinurid lobsters mostly originate from Australia, New Zealand, Cuba, Brazil, South Africa, USA and Mexico. The annual world catch of lobsters is 2, 33,825 t (2005) valued at US $ 2108 million. Total landing of spiny lobsters is 71925 t (2004). Most world fisheries are either fully exploited, or overexploited. Annual landing of lobsters in India was 1539 t (2007), which averaged about 2200 t in 1990’s. Lobsters have excellent market demand and price and especially live lobsters are the most preferred. Annual export was to the tune of Rs.53 crores during 2003-04. Lobsters are exported in different forms; frozen tails, whole frozen, whole-chilled, whole-cooked and frozen, lobster meat and as in live form. Overseas customers are ready to pay more for fresh seafood. Live lobster export increased from 1.3% during 1993- 94 to 12.7% during 2003-04 periods. -

Spiny Lobster (Panulirus Sp.) Life Cycle – a Review and Fisheries Management Implications
Research Notes… Spiny Lobster (Panulirus sp.) Life Cycle – a review and fisheries management implications Léo Barret Abstract The fishery for spiny lobster around the inner granitic islands of the Seychelles has been closed for two consecutive years (2017-2019). Declining trends in indicators of abundance and the loss of coral reef habitats led to the SeyCCAT funded lobster project, aimed at establishing a science-based restoration of commercially important spiny lobster habitats to help develop a sustainable fishery – a project partnership between the University of Seychelles (UniSey), the Seychelles Fishing Authority (SFA), and the Marine Conservation Society Seychelles (MCSS). Spiny lobsters have a complex mero-planktonic lifecycle from a larva living in the open sea to an adult living on the sea floor. The management of the fishery requires a good understanding on all the stages of the spiny lobster life cycle. In this context, the objective of this paper is to provide a short review of the spiny lobster (Panulirus sp.) life cycle and the ecological and fisheries management implications. Introduction (a Seychelles perspective) Spiny lobsters (Palinuridae) are one of the most valuable commercial seafood species globally; for example in Australia, lobster is the country’s most valuable fishery in terms of both overall production and value of export, with a Gross Value of Production of $610 million AUD in 2014 (Plagányi et al., 2018). The FAO annual catch data of spiny (Palinuridae) and clawed (Nephropidae) lobsters has been steadily increasing over the past few decades and the 2017 global catch estimate was 315,469 t (FAO, 2019). Unlike the global trend, Seychelles’ lobster fishery catch has decreased over the past decade, with some of the major challenges being limited recruitment, poaching, and rapid ecosystem change, such as the loss of coral reef habitats due to coral bleaching events. -

Histology and Ultrastructure of Male Reproductive System of the Indian Spiny Lobster Panulirus Homarus (Decapoda: Palinuridae)
Histology and ultrastructure of male reproductive system of the Indian Spiny lobster Panulirus homarus (Decapoda: Palinuridae) S. Lakshmi Pillai1*, M. Nasser2 & N. K. Sanil1 1. Central Marine Fisheries Research Institute, P.B.No.1603, Ernakulam North, P.O., Kochi-18, India; [email protected], [email protected] 2. Department of Zoology, University of Calicut, Thenipalam, Kerala, India; [email protected] * Correspondence Received 24-IV-2013. Corrected 10-X-2013. Accepted 15-XI-2013. Abstract: The spiny lobster Panulirus homarus, distributed along the Southeast and Southwest coasts of India, is an important commercial species having mariculture potential. Despite its importance, the structural and ultrastructure features of male gonads from this species have received scarce attention. Hence this study was aimed to describe the male reproductive tract of the species, using standard histological and electron micros- copy techniques. Gonads from 94 specimens of P. homarus ranging in carapace length 37mm-92mm from Vizhinjam (Southwest coast of India.) were obtained and processed for the study (Histology-70 numbers & ultrastructure-24 numbers). The male reproductive system consists of paired testis and vas deferens located in the cephalo-thoracic region. Macroscopically, the reproductive tract was observed in lobsters >35mm carapace length. In immature testis, spermatogonia were seen which measured 6.9-13.8µm in diameter and in the mature testis primary (5.4-5.9µm) and secondary spermatocytes (2.8-3µm) and spermatids (2.2-2.4µm) were pres- ent. Each vas deferens consists of proximal and distal portions. The spermatophoric mass begins formation in the proximal vas deferens. In the distal vas deferens the spermatophoric mass containing the spermatozoa are arranged in packets towards the periphery by the gelatinous matrix produced by the typhlosole. -

Tropical Agricultural Science
Pertanika J. Trop. Agric. Sc. 42 (1): 209 - 223 (2019) TROPICAL AGRICULTURAL SCIENCE Journal homepage: http://www.pertanika.upm.edu.my/ Length-weight and Morphometric Analysis of Mud Lobster, Thalassina anomala from Sarawak, Malaysia Zakaria Nur-Nadiah1, Masnindah Malahubban1, Sharida Fakurazi2, Sie Chuong Wong3 and Amy Halimah Rajaee1* 1Department of Animal Science and Fishery, Faculty of Agriculture and Food Sciences, Universiti Putra Malaysia Bintulu Sarawak Campus, 97008 Bintulu, Sarawak, Malaysia 2Department of Human Anatomy, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia 3Department of Basic Science and Engineering, Faculty of Agriculture and Food Sciences, Universiti Putra Malaysia Bintulu Sarawak Campus, 97008 Bintulu, Sarawak, Malaysia ABSTRACT Mud lobsters (Thalassina spp.) are nocturnal organisms, belong to Order Decapoda which are lesser known and least studied although their presence were widely distributed across the Indo-West Pacific Region. Mud lobster is considered as an important organism in the mangrove ecosystem for its burrowing activities and the role of its mounds or burrows as home to other animals. All mud lobster samples collected from four distinct regions in Sarawak (Kuala Tatau, Kuala Balingian, Sarikei and Lingga) were identified asThalassina anomala based on the morphological characteristics. Morphometric variations between sexes of T. anomala were examined. Sexual dimorphism can be observed where males had significantly longer carapace length, left chelae propodus length and larger left chelae propodus width compared to females (p<0.05). Meanwhile, sexual dimorphism can also be seen in the abdominal width, in which it was significantly larger in females, for both absolute values (p<0.01) and also the relative values (p<0.001). -

5. Index of Scientific and Vernacular Names
click for previous page 277 5. INDEX OF SCIENTIFIC AND VERNACULAR NAMES A Abricanto 60 antarcticus, Parribacus 209 Acanthacaris 26 antarcticus, Scyllarus 209 Acanthacaris caeca 26 antipodarum, Arctides 175 Acanthacaris opipara 28 aoteanus, Scyllarus 216 Acanthacaris tenuimana 28 Arabian whip lobster 164 acanthura, Nephropsis 35 ARAEOSTERNIDAE 166 acuelata, Nephropsis 36 Araeosternus 168 acuelatus, Nephropsis 36 Araeosternus wieneckii 170 Acutigebia 232 Arafura lobster 67 adriaticus, Palaemon 119 arafurensis, Metanephrops 67 adriaticus, Palinurus 119 arafurensis, Nephrops 67 aequinoctialis, Scyllarides 183 Aragosta 120 Aesop slipper lobster 189 Aragosta bianca 122 aesopius, Scyllarus 216 Aragosta mauritanica 122 affinis, Callianassa 242 Aragosta mediterranea 120 African lobster 75 Arctides 173 African spear lobster 112 Arctides antipodarum 175 africana, Gebia 233 Arctides guineensis 176 africana, Upogebia 233 Arctides regalis 177 Afrikanische Languste 100 ARCTIDINAE 173 Agassiz’s lobsterette 38 Arctus 216 agassizii, Nephropsis 37 Arctus americanus 216 Agusta 120 arctus, Arctus 218 Akamaru 212 Arctus arctus 218 Akaza 74 arctus, Astacus 218 Akaza-ebi 74 Arctus bicuspidatus 216 Aligusta 120 arctus, Cancer 217 Allpap 210 Arctus crenatus 216 alticrenatus, Ibacus 200 Arctus crenulatus 218 alticrenatus septemdentatus, Ibacus 200 Arctus delfini 216 amabilis, Scyllarus 216 Arctus depressus 216 American blunthorn lobster 125 Arctus gibberosus 217 American lobster 58 Arctus immaturus 224 americanus, Arctus 216 arctus lutea, Scyllarus 218 americanus, -

Reproductive Biology of the Female Shovel-Nosed Lobster Thenus Unimaculatus (Burton and Davie, 2007) from North-West Coast of India
Indian Journal of Geo-Marine Sciences Vol. 43(6), June 2014, pp. 927-935 Reproductive biology of the female shovel-nosed lobster Thenus unimaculatus (Burton and Davie, 2007) from north-west coast of India Joe K. Kizhakudan Madras Research Centre of Central Marine Fisheries Research Institute 75, Santhome High Road, R.A. Puram, Chennai-600 028, Tamil Nadu, India [E-mail: [email protected]] Received 10 September 2012; revised 17 January 2013 Reproductive biology of the female shovel-nosed lobster Thenus unimaculatus from the north-west coast of India was studied from samples obtained from the fishery along Gujarat coast. Development of secondary sexual characters, gonadal maturation, size at sexual maturity, fecundity and Gonadosomatic Index (GSI) were assessed. Development of ovigerous setae on abdominal pleopods and size of gonopore were identified as secondary sexual characters indicative of the maturation state of the lobster. Onset of maturity in females is marked by the development of ovigerous setae. There are no mating windows (ventral sternite region) in female T. unimaculatus. Female reproductive tract in T. unimaculatus comprises of ovaries, oviducts and genital opening. Ovarian development in T. unimaculatus could be classified into six stages - Immature, Early Maturing, Late Maturing/Mature, Ripe, Spawning and Spent/Recovery. The process of ovarian maturation in T. unimaculatus could be traced through three distinct phases depending on the extent of yolk deposition – Pre-vitellogenesis, Primary vitellogenesis and Secondary vitellogenesis. Morphological, physiological and functional maturity is almost simultaneous in T. unimaculatus, with the size at first maturity in females attained at 61-65 mm CL. Size at onset of sexual maturity, judged from the 25% success rate in development of different sexual characters is 51-55 mm CL. -

The 11Th International Conference and Workshop on Lobster Biology and Management, Hosted by the University of Maine and Boston University in Portland, Maine
The University of Maine DigitalCommons@UMaine Maine Sea Grant Publications Maine Sea Grant 6-2017 The 11th nI ternational Conference and Workshop on Lobster Biology and Management Richard Wahle University of Maine, School of Marine Sciences, Darling Marine Center, Walpole, ME 04573, [email protected] Kari L. Lavalli Boston University, College of General Studies, Division of Natural Sciences & Mathematics, 871 Commonwealth Avenue, Boston, MA 02215, [email protected] Follow this and additional works at: https://digitalcommons.library.umaine.edu/seagrant_pub Part of the Aquaculture and Fisheries Commons Repository Citation Wahle, Richard and Lavalli, Kari L., "The 11th nI ternational Conference and Workshop on Lobster Biology and Management" (2017). Maine Sea Grant Publications. 144. https://digitalcommons.library.umaine.edu/seagrant_pub/144 This Conference Proceeding is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Maine Sea Grant Publications by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. PROGRAM & ABSTRACTS PROGRAM & ABSTRACTS OUR SPONSORS OUR SPONSORS The 11th ICWL Steering Committee gratefully acknowledges the support of the following The 11th ICWL Steering Committee gratefully acknowledges the support of the following (continued on back inside cover): (continued on back inside cover): Host Host Sponsors Sponsors Gold Silver Gold Silver Sponsor Sponsors Sponsor Sponsors Bronze Bronze Sponsors Sponsors Office of Research Office of Research Custom Custom Sponsors Sponsors School of Marine Sciences School of Marine Sciences 11TH INTERNATIONAL CONFERENCE & WORKSHOP ON LOBSTER BIOLOGY & MANAGEMENT Dear Participants, On behalf of the Steering Committee, we are pleased to welcome you to the 11th International Conference and Workshop on Lobster Biology and Management, hosted by the University of Maine and Boston University in Portland, Maine.