A Landscape-Level Assessment of Composition, Structural Heterogeneity and Distribution Pattern of Trees in Temperate Forest of Kashmir Himalaya, India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Improvement of Seed Germination in Three Important Conifer Species by Gibberellic Acid (GA3)
Volume 11(2) Improvement of seed germination in three important conifer species by Gibberellic acid (GA3). Improvement of seed germination in three important conifer species by Gibberellic acid (GA3). B. S. Rawat1, C. M. Sharma2 and S. K. Ghildiyal3 Department of Forestry, Post Box # 76, HNB Garhwal University, Srinagar Garhwal-246 174 (Uttaranchal) 1. [email protected] 2. [email protected] [email protected] December 2006 Download at: http://www.lyonia.org/downloadPDF.php?pdfID=283.486.1 Improvement of seed germination in three important conifer species by Gibberellic acid (GA3). Abstract Results pertaining to the germination percentage of pre-soaked seeds in a series of temperature regimes viz., 100C, 150C, 200C and 250C have revealed significant increase among seed sources in each of the three conifer species of Garhwal Himalaya. Soaking of the seeds for 24 hours in GA3 solution had shown maximum germination in A. pindrow (45.0±4.19%), C. torulosa (57.0±3.40%) and P. smithiana (56±6.01%) as compared to untreated (control) seeds. It has also been observed that GA3 treatment caused an appreciable shortening of the germination period by 10 days. Therefore, seeds of these commercially important tree species should be pre-treated particularly with GA3 for 24 hours for getting enhanced germination. It is important to point out here that the seeds of each of the three species reflect poor germination in nature due to snow cover, seed decay, prevalence of excess water and lack of maintenance, however, because of increasing demand for large quantities of tree seeds for reforestation programmes, pre-sowing treatments are useful to improve the rate and percentage of germination. -

Variation in Soil CO2 Efflux in Pinus Wallichiana and Abies Pindrow
rch: O ea pe es n A R t c s c e e Sundarapandian and Dar, Forest Res 2013, 3:1 r s o s Forest Research F DOI: 10.4172/2168-9776.1000116 Open Access ISSN: 2168-9776 Research Article Open Access Variation in Soil CO2 Efflux in Pinus Wallichiana and Abies Pindrow Temperate Forests of Western Himalayas, India SM Sundarapandian* and Javid Ahmad Dar Department of Ecology and Environmental Sciences, School of life Sciences, Pondicherry University, Puducherry, India Abstract Soil CO2 efflux was measured by alkali absorption method from April to December 2012 in two different forest types, i.e., Pinus wallichiana and Abies pindrow, with three replicate plots in each forest type. Soil CO2 efflux was found maximum in July and minimum in December in both the forest types. Significantly (P<0.001) greater soil CO2 efflux was measured inPinus wallichiana forest compared to Abies pindrow forest throughout the study period. The -2 -1 range of soil CO2 efflux (mg CO2 m hr ) from the soil was 126-427 in Abies pindrow forest and 182-646 in Pinus wallichiana forest. Soil CO2 efflux showed greater values in Pinus wallichiana forest than Abies pindrow forest, which could be attributed to greater tree density, tree biomass, shrub density, shrub biomass, forest floor litter and moisture. Soil CO2 efflux also showed significant positive relationship with air temperature. In addition to that the altitudinal difference may be one of the reasons for variation in soil CO2 efflux between the two forest types. This result also indicates that at higher altitude even a small difference in elevation (100 m) alter the functional attributes of the ecosystem. -
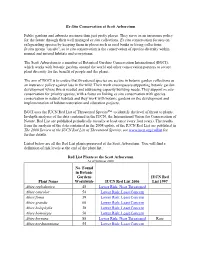
IUCN Red List of Threatened Species™ to Identify the Level of Threat to Plants
Ex-Situ Conservation at Scott Arboretum Public gardens and arboreta are more than just pretty places. They serve as an insurance policy for the future through their well managed ex situ collections. Ex situ conservation focuses on safeguarding species by keeping them in places such as seed banks or living collections. In situ means "on site", so in situ conservation is the conservation of species diversity within normal and natural habitats and ecosystems. The Scott Arboretum is a member of Botanical Gardens Conservation International (BGCI), which works with botanic gardens around the world and other conservation partners to secure plant diversity for the benefit of people and the planet. The aim of BGCI is to ensure that threatened species are secure in botanic garden collections as an insurance policy against loss in the wild. Their work encompasses supporting botanic garden development where this is needed and addressing capacity building needs. They support ex situ conservation for priority species, with a focus on linking ex situ conservation with species conservation in natural habitats and they work with botanic gardens on the development and implementation of habitat restoration and education projects. BGCI uses the IUCN Red List of Threatened Species™ to identify the level of threat to plants. In-depth analyses of the data contained in the IUCN, the International Union for Conservation of Nature, Red List are published periodically (usually at least once every four years). The results from the analysis of the data contained in the 2008 update of the IUCN Red List are published in The 2008 Review of the IUCN Red List of Threatened Species; see www.iucn.org/redlist for further details. -

<I>Pinus Wallichiana</I>
ISSN (print) 0093-4666 © 2012. Mycotaxon, Ltd. ISSN (online) 2154-8889 MYCOTAXON http://dx.doi.org/10.5248/121.225 Volume 121, pp. 225–232 July–September 2012 Suillus flavidus and its ectomycorrhizae with Pinus wallichiana in Pakistan S. Sarwar*, A.N. Khalid, M. Hanif & A.R. Niazi Department of Botany, University of the Punjab, Quaid-e-Azam Campus, Lahore, 54590, Pakistan * Correspondence to: [email protected] Abstract — Suillus flavidus (Boletales, Suillaceae) was found associated with Pinus wallichiana during a survey of macrofungi from moist coniferous forests of Pakistan. Both the fruiting body and ectomycorrhizae were characterized morpho-anatomically as well as by molecular analysis. This fungus is a new record for Pakistan and its ectomycorrhizae with Pinus wallichiana are described for the first time by molecular analysis. Key words —boletes, ITS, mantle, PCR, rDNA Introduction Coniferous forests of Pakistan are located at an elevation of 1373 to 3050 m a.s.l. and are characterized by luxuriant growth of trees such as Abies pindrow, Cedrus deodara, Picea smithiana, Pinus roxburghii, P. wallichiana, and Taxus wallichiana. Among these conifers, some deciduous trees and shrubs of different species also occur (Hussain 1995). Another important feature of these forests is the high level of rainfall during summer (July–August). High rainfall and temperature make an environment suitable for the growth of mushrooms. Most of these fungi form mutualistic symbiotic associations with forest trees in the form of ectomycorrhizae that facilitate tree growth through enhanced nutrient absorption and protection of roots from root pathogens (Marx 1991). Suillus Gray, a genus with approximately 50 species (Kirk et al. -

Tree-Ring Chronologies of Picea Smithiana (Wall.) Boiss., and Its Quantitative Vegetational Description from Himalayan Range of Pakistan
Pak. J. Bot., 37(3): 697-707, 2005. TREE-RING CHRONOLOGIES OF PICEA SMITHIANA (WALL.) BOISS., AND ITS QUANTITATIVE VEGETATIONAL DESCRIPTION FROM HIMALAYAN RANGE OF PAKISTAN MOINUDDIN AHMED AND SYED HUMAIR NAQVI Department of Botany, Federal Urdu University of Arts, Science and Technology, Gulshan-e-Iqbal Campus, University Road, Karachi. 75270, Pakistan. Abstract Modern Dendrochronological techniques were used in 5 stands of moist temperate and dry temperate areas in Pakistan. Out of 91 cores from 60 trees of Picea smithiana (Wall.) Boiss., sampled where cross dating was possible among 48 cores. Dated chronologies from 1422 to 1987 AD were obtained. However, common period of all chronologies 1770 to 1850 A.D. is presented. Chronologies and sample statistics are described. These chronologies show from 17% to 33% variance (“Y” in ANOVA) due to climate. Dry temperate sites show low autocorrelation as compared to moist temperate sites. Due to small sample size, no statistical correlation was observed between community and dendrochronological attributes. However, community attributes gave some idea to select better sites for dendrochronological investigations. It is suggested that despite difference in climatic zones and chronologies, trees show some similar pattern of ring-width. Hence, Picea smithiana (Wall.) Boiss., could be used for dendroclimatological investigations. It is also suggested that detailed sampling is required to present strong database. Introduction Ahmed (1987, 1989) explained the scope of dendrochronology in Pakistan, and mentioned suitable sites and tree species, which could be used in tree-ring analysis. He also presented modern tree-ring chronologies of Abies pindrow Royle from Himalayan region of Pakistan. A dendrochronological approach to estimate age and growth pattern of various species and dendrochronological potential of a few tree species from the Himalayan region of Pakistan was described by Ahmed & Sarangezai (1991, 1992). -

Diversity and Ethnobotanical Importance of Pine Species from Sub-Tropical Forests, Azad Jammu and Kashmir
Journal of Bioresource Management Volume 7 Issue 1 Article 10 Diversity and Ethnobotanical Importance of Pine Species from Sub-Tropical Forests, Azad Jammu and Kashmir Kishwar Sultana PMAS-Arid Agriculture University, Rawalpindi, Pakistan Sher Wali Khan Department of Biological Sciences, Karakoram International University, Gilgit, Pakistan, [email protected] Safdar Ali Shah Khyber Pakhtunkhwa (KP) Wildlife Department, Peshawar, Pakistan Follow this and additional works at: https://corescholar.libraries.wright.edu/jbm Part of the Biodiversity Commons, Botany Commons, and the Other Ecology and Evolutionary Biology Commons Recommended Citation Sultana, K., Khan, S. W., & Shah, S. A. (2020). Diversity and Ethnobotanical Importance of Pine Species from Sub-Tropical Forests, Azad Jammu and Kashmir, Journal of Bioresource Management, 7 (1). DOI: https://doi.org/10.35691/JBM.0202.0124 ISSN: 2309-3854 online This Article is brought to you for free and open access by CORE Scholar. It has been accepted for inclusion in Journal of Bioresource Management by an authorized editor of CORE Scholar. For more information, please contact [email protected]. Diversity and Ethnobotanical Importance of Pine Species from Sub-Tropical Forests, Azad Jammu and Kashmir © Copyrights of all the papers published in Journal of Bioresource Management are with its publisher, Center for Bioresource Research (CBR) Islamabad, Pakistan. This permits anyone to copy, redistribute, remix, transmit and adapt the work for non-commercial purposes provided the original work and source is appropriately cited. Journal of Bioresource Management does not grant you any other rights in relation to this website or the material on this website. In other words, all other rights are reserved. -

Breeding and Genetic Resources of Five-Needle Pines: Growth, Adaptability
Genetic Variation in Blue Pine and Applications for Tree Improvement in Pakistan, Europe and North America Shams R. Khan Abstract—Stands of blue pine (P. wallichiana A.B. Jacks. syn. P. Khan (1972), along with several earlier investigators includ- griffithii McClelland) are highly diverse throughout its range of ing Brandis (1906), Osmaston (1927), and Shebbeare (1934), distribution in the Himalayan Mountains where the species grows have recognized this variable site distribution of the species under varying geographic, climatic, and edaphic conditions. The occurring in several countries of the region. Pure and mixed species occurs in two distinctly different ecotypes (mesic monsoon patches at varying altitudes are found, but the species grows and dry nonmonsoon), and strict avoidance of germplasm transfer well at an optimum elevation of 2,000-2,500 M. Although between the ecotypes is necessary for survival and productivity in this pine occurs over a wide altitudinal range, there is no Pakistan, India, and Nepal. The role of these ecotypes in enhancing evidence of altitudinal races that could be given subspecific productivity and in establishing large-scale plantations resistant to or specific taxonomic ranking. blister rust is presented and compared with plantations in India and This species has been known by a number of scientific Bhutan. An alternate management strategy to establishing a pure names since first described. The taxonomy of blue pine has species stand is to interplant with other native conifers. Testing of been a subject of controversy, probably corresponding to blue pine in other countries is discussed, notably the superior the diversity in the species on the wide range of ecotypes performance of blue pine hybrids in the USA at specific sites, which where it occurs. -

Impact of Silvicultural System on Natural Regeneration in Western Himalayan Moist Temperate Forests of Pakistan
Journal of Forest Science, 67, 2021 (3): 101–112 Original Paper https://doi.org/10.17221/124/2020-JFS Impact of silvicultural system on natural regeneration in Western Himalayan moist temperate forests of Pakistan Javed Iqbal 1,2* 1Department of Silviculture, Faculty of Forestry and Wood Sciences, Czech University of Life Sciences Prague, Prague, Czech Republic 2Department of Forestry, Shaheed Benazir Bhutto University, Sheringal, Upper Dir, Khyber Pakhtunkhwa, Pakistan *Corresponding author: [email protected]; [email protected] Citation: Iqbal J. (2021): Impact of silvicultural system on natural regeneration in Western Himalayan moist temperate forests of Pakistan. J. For. Sci., 67: 101–112. Abstract: Site conditions (topography, aspect, moisture availability, humus thickness, light exposure, and grazing ac- tivities) play a vital role in the germination and regeneration process. The research was conducted in the Himalayan moist temperate forest. The research site was divided based on the silvicultural system (group selection system and single-tree selection system) into 148 plots and 150 plots, respectively. The group selection system was examined on the site of 2 ha which was clear-felled under a project in the 1980's. The present study examined the impact of silvi- cultural systems on regeneration. The frequency table was used, and relative frequency was calculated for the species and silvicultural system, density per m2 was also calculated. Diversity indices were calculated through taxa, dominance, Simpson’s index, Shannon index, evenness, equitability, and fisher alpha. Ten taxa were found in both silvicultural sys- tems, with individual repetition of 17 and 15 taxa, respectively. Group selection is more compact visibly as compared to the single-tree selection system. -

Chemosystematics of Some Conifers of India
Proc. Indian Acad. Sci. (Plant Sci.), Vol. 99, No. 3, June 1989, pp. 253-258. O Printed in India. Chemosystematics of some conifers of India SHEEJA M JOHN, M DANIEL and S D SABNIS Phytochemistry Laboratory, Department of Botany, Faculty of Science, M S University of Baroda, Baroda 390 002, India. MS received 6 June 1988; revised 13 December 1988 Abstract. Flavonoids and related phenolics in the leaves of 14 conifers were studied and their relevance in the taxonomy of the group was assessed. It is found that biflavones were absent from the Pinaceae and Taxaceae. The former family was also devoid of flavones and possessed a low frequency of incidence of flavonols. The entire flavonoid system was absent in Pinus. In the light of these chemical evidences, the identities of the Pinaceae and Taxaceae as separate orders Pinales and Taxales, are defended. The rest of the conifers are grouped in a new order, the Cupressales. The merger of Taxodiaceae and Cupressaceae is strongly opposed. Incorporating all the taxonomic evidences a new scheme of classification is proposed. Keywords. Chemotaxonomy;conifers; Pinales; Taxales; Cupressales; biflavones. 1. lntroduction The conifers are the representative gs'mnosperms of the present flora and constitute more than three fourths of the gymnosperms. In India 12-15 genera of conifers, containing more than 20 species, are available, mostly distributed in the Himalaya. Coulter and Chamberlain (1917) recognised two families of conifers, the Pinaceae and the Taxaceae. The Pinaceae possess distinct female strobili in which the overlapping scales protect the ovules. This family is divided into 4 tribes: (i) Abietineae, (ii) Taxodineae, (iii) Cupressineae and (iv) Araucarineae. -

<I>Picea Smithiana</I>
MYCOTAXON Volume 107, pp. 73–80 January–March 2009 Ectomycorrhizae between Amanita rubescens and Himalayan spruce (Picea smithiana) from Pakistan A. R. Niazi, S. H. Iqbal & A. N. Khalid [email protected] Department of Botany, University of the Punjab Quaid-e-Azam Campus, Lahore, 54590, Pakistan Abstract — Field ectomycorrhizae of Amanita rubescens with Picea smithiana are described for the first time. Ectomycorrhizal roots were sampled beneath the sporocarp from the rhizosphere of the host plant. The sporocarps and ectomycorrhizae were characterized morphologically and anatomically. The most important characters of the ectomycorrhizae are a monopodial mycorrhizal system, white to cream colour of the mantle, hyaline emanating hyphae, and thick hairy rhizomorphs emerging mostly from restricted points. All mantle layers are plectenchymatous. Hyphae lack clamp connections forming rings. Rhizomorphs are composed of thick central hyphae without septa. The diameter of the rhizomorphs is narrower than other known ECM of Amanita spp. This association is reported for the first time from Pakistan. Key words — Amanitaceae, ectomycorrhizogenous, Mukshpuri, Pinaceae Introduction Himalayan spruce (Picea smithiana (Wall.) Boiss.) is native to western Himalaya and adjacent mountains from northeast Afghanistan to central Nepal. It grows at altitudes of 2400–3600 m a.s.l. in forests together with Cedrus deodara (Roxb.) D. Don, Pinus wallichiana A.B. Jacks., and Abies pindrow (Royle ex D. Don) Royle. It is valued for its use in the pulp industry as a source of paper. Although it is of marginal use as fuel, it yields a fairly good charcoal (Hill 1952). In Pakistan Himalayan spruce is found in northern Pakistan (Kurram Agency, Chitral, Swat and Gilgit eastwards), Murree hills (Nathia Gali, north slope of Mukshpuri) and Kashmir (Stewart 1972). -

Influence of Climate on Radial Growth of Abies Pindrow in Western Nepal Himalaya
Influence of climate on radial growth of Abies pindrow in Western Nepal Himalaya U.K. Thapa1*, S. K. Shah2, N. P. Gaire3, D. R. Bhuju4, A. Bhattacharyya2 and G. S. Thagunna1 This study aims to understand the influence of climate on radial growth of Abies pindrow growing in the plateau of mixed forest in Khaptad National Park in Western Nepal Himalaya. Based on the dated tree-ring samples, 362-year long tree-ring width chronology was developed dating back to 1650. The studied taxa of this region was found to have dendroclimatic potentiality that was evident from the chronology statistics calculated. The tree-ring chronology was correlated with climate (temperature and precipitation) data to derive the tree-growth climate relationship. The result showed significant negative relationship with March-May temperature and positive relationship with March-May precipitation. This indicates that the availability of moisture is the primary factor in limiting the tree growth. Key words: Abies pindrow, tree-ring, climatic influence, Khaptad National Park, pre-monsoon arth’s climate has never been static and In the last decade, a number of dendro has shown great variability since its origin, chronological studies from Nepal Himalaya have Ethe recent change, however, is accelerated been conducted. However, most of these studies by enhanced greenhouse effect causing abrupt are from Central and Eastern Nepal Himalaya temperature rise and unpredictable patterns (Cook et al., 2003; Bhuju et al., 2010; Chhetri of precipitation (Houghton, 2004). Feedbacks and Thapa, 2010; Gaire et al., 2011; Dawadi et of climate change are reflected in several al., 2013). There is a dearth of such works in components of earth including air, water, ice, land Western Nepal Himalaya, though this part of and vegetation which have distinctive response Nepal Himalaya has huge potential for tree-ring time to the changing climate and the natural research (Suzuki, 1990; Bhattacharyya et al., archives like tree rings, ice cores, sediments, 1992). -

Full Article
INTERNATIONAL JOURNAL OF CONSERVATION SCIENCE ISSN: 2067-533X Volume 5, Issue 4, October-December 2014: 527-534 www.ijcs.uaic.ro GROWING STOCK OF VARIOUS BROAD-LEAVED AND CONIFER FORESTS OF GARHWAL HIMALAYA Suchita DIMRI∗, Pratibha BALUNI, Chandra Mohan SHARMA Department of Botany, HNB Garhwal University, Srinagar Garhwal-246174, India Abstract The present study was undertaken to assess the Growing Stock and Population Structure of ten temperate forest types of Garhwal Himalaya between 1200-3000m asl. Five sample-plots of 0.1 ha were randomly laid out at two sites (I and II) in each forest type to estimate the GSVD (Growing Stock Volume Density) (by using appropriate volume tables and volume equations). The GSVD values in different forest types oscillated between 198.78 m3/ha (for Quercus glauca) to 907.74 m3/ha (for Cedrus deodara forest). The density-diameter distribution was calculated to understand the pattern of Regeneration status of the forest type. Quercus floribunda forest type was recorded as the most dense forest with 710ind/ha (Site I) and 790 ind/ha (Site II). Analysis of Growing stock and density of particular forest type plays an important role in forest management and proper strategies for the conservation of the forests. GSVD estimation leads to quantification of biomass, which in turn is essential to assess the amount of carbon stored in the forest. The estimation of GSVD has, therefore assumed significance in estimating climate change scenario. Keywords: Biomass; Temperate forests; Carbon storage; Density. Introduction Forests are among the most productive terrestrial ecosystems, which along with their long-lived woody character, makes them attractive for climate change mitigation [1].