Seed Transfer of Woody Shrubs in Alberta –
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Willows of Interior Alaska
1 Willows of Interior Alaska Dominique M. Collet US Fish and Wildlife Service 2004 2 Willows of Interior Alaska Acknowledgements The development of this willow guide has been made possible thanks to funding from the U.S. Fish and Wildlife Service- Yukon Flats National Wildlife Refuge - order 70181-12-M692. Funding for printing was made available through a collaborative partnership of Natural Resources, U.S. Army Alaska, Department of Defense; Pacific North- west Research Station, U.S. Forest Service, Department of Agriculture; National Park Service, and Fairbanks Fish and Wildlife Field Office, U.S. Fish and Wildlife Service, Department of the Interior; and Bonanza Creek Long Term Ecological Research Program, University of Alaska Fairbanks. The data for the distribution maps were provided by George Argus, Al Batten, Garry Davies, Rob deVelice, and Carolyn Parker. Carol Griswold, George Argus, Les Viereck and Delia Person provided much improvement to the manuscript by their careful editing and suggestions. I want to thank Delia Person, of the Yukon Flats National Wildlife Refuge, for initiating and following through with the development and printing of this guide. Most of all, I am especially grateful to Pamela Houston whose support made the writing of this guide possible. Any errors or omissions are solely the responsibility of the author. Disclaimer This publication is designed to provide accurate information on willows from interior Alaska. If expert knowledge is required, services of an experienced botanist should be sought. Contents -

Chrysomela 43.10-8-04
CHRYSOMELA newsletter Dedicated to information about the Chrysomelidae Report No. 43.2 July 2004 INSIDE THIS ISSUE Fabreries in Fabreland 2- Editor’s Page St. Leon, France 2- In Memoriam—RP 3- In Memoriam—JAW 5- Remembering John Wilcox Statue of 6- Defensive Strategies of two J. H. Fabre Cassidine Larvae. in the garden 7- New Zealand Chrysomelidae of the Fabre 9- Collecting in Sholas Forests Museum, St. 10- Fun With Flea Beetle Feces Leons, France 11- Whither South African Cassidinae Research? 12- Indian Cassidinae Revisited 14- Neochlamisus—Cryptic Speciation? 16- In Memoriam—JGE 16- 17- Fabreries in Fabreland 18- The Duckett Update 18- Chrysomelidists at ESA: 2003 & 2004 Meetings 19- Recent Chrysomelid Literature 21- Email Address List 23- ICE—Phytophaga Symposium 23- Chrysomela Questionnaire See Story page 17 Research Activities and Interests Johan Stenberg (Umeå Univer- Duane McKenna (Harvard Univer- Eduard Petitpierre (Palma de sity, Sweden) Currently working on sity, USA) Currently studying phyloge- Mallorca, Spain) Interested in the cy- coevolutionary interactions between ny, ecological specialization, population togenetics, cytotaxonomy and chromo- the monophagous leaf beetles, Altica structure, and speciation in the genus somal evolution of Palearctic leaf beetles engstroemi and Galerucella tenella, and Cephaloleia. Needs Arescini and especially of chrysomelines. Would like their common host plant Filipendula Cephaloleini in ethanol, especially from to borrow or exchange specimens from ulmaria (meadow sweet) in a Swedish N. Central America and S. America. Western Palearctic areas. Archipelago. Amanda Evans (Harvard University, Maria Lourdes Chamorro-Lacayo Stefano Zoia (Milan, Italy) Inter- USA) Currently working on a phylogeny (University of Minnesota, USA) Cur- ested in Old World Eumolpinae and of Leptinotarsa to study host use evolu- rently a graduate student working on Mediterranean Chrysomelidae (except tion. -

Plant Materials Tech Note
United States Department of Agriculture NATURAL RESOURCES CONSERVATION SERVICE Plant Materials Plant Materials Technical Note No. MT-33 September 1999 PLANT MATERIALS TECH NOTE DESCRIPTION, PROPAGATION, AND USE OF SILVERBERRY Elaeagnus commutata. Introduction: Silverberry Elaeagnus commutata Bernh. ex. Rydb is a native shrub with potential use in streambank stabilization, wildlife habitat, windbreaks, and naturalistic landscaping projects. The purpose of this bulletin is to transfer information on the identification, culture, and proper use of this species. FIGURE 1 FRUIT STEM FOLIAGE I. DESCRIPTION Silverberry is a multi-stemmed, deciduous shrub ranging from 1.5 to 3.6 m (5 to 12 ft.) tall. In Montana, heights of 1.5 to 2.4 m (5 to 8 ft.) are most common. It has an erect, upright habit with slender and sometimes twisted branches. The new stems are initially a light to medium brown color, the bark becoming dark gray, but remaining smooth, with age. The leaves are deciduous, alternate, 38 to 89 mm (1.5 to 3.5 in.) long and 19 to 38 mm (0.75 to 1.5 in.) wide (see Figure 1). The leaf shape is described as oval to narrowly ovate with an entire leaf margin. Both the upper and lower leaf surfaces are covered with silvery white scales, the bottom sometimes with brown spots. The highly fragrant, yellow flowers are trumpet-shaped (tubular), approximately 13 mm (0.5 in.) in length, and borne in the leaf axils in large numbers in May or June. The fruit is a silvery-colored, 7.6 mm (0.3 in.) long, egg-shaped drupe that ripens in September to October. -

Plant List by Hardiness Zones
Plant List by Hardiness Zones Zone 1 Zone 6 Below -45.6 C -10 to 0 F Below -50 F -23.3 to -17.8 C Betula glandulosa (dwarf birch) Buxus sempervirens (common boxwood) Empetrum nigrum (black crowberry) Carya illinoinensis 'Major' (pecan cultivar - fruits in zone 6) Populus tremuloides (quaking aspen) Cedrus atlantica (Atlas cedar) Potentilla pensylvanica (Pennsylvania cinquefoil) Cercis chinensis (Chinese redbud) Rhododendron lapponicum (Lapland rhododendron) Chamaecyparis lawsoniana (Lawson cypress - zone 6b) Salix reticulata (netleaf willow) Cytisus ×praecox (Warminster broom) Hedera helix (English ivy) Zone 2 Ilex opaca (American holly) -50 to -40 F Ligustrum ovalifolium (California privet) -45.6 to -40 C Nandina domestica (heavenly bamboo) Arctostaphylos uva-ursi (bearberry - zone 2b) Prunus laurocerasus (cherry-laurel) Betula papyrifera (paper birch) Sequoiadendron giganteum (giant sequoia) Cornus canadensis (bunchberry) Taxus baccata (English yew) Dasiphora fruticosa (shrubby cinquefoil) Elaeagnus commutata (silverberry) Zone 7 Larix laricina (eastern larch) 0 to 10 F C Pinus mugo (mugo pine) -17.8 to -12.3 C Ulmus americana (American elm) Acer macrophyllum (bigleaf maple) Viburnum opulus var. americanum (American cranberry-bush) Araucaria araucana (monkey puzzle - zone 7b) Berberis darwinii (Darwin's barberry) Zone 3 Camellia sasanqua (sasanqua camellia) -40 to -30 F Cedrus deodara (deodar cedar) -40 to -34.5 C Cistus laurifolius (laurel rockrose) Acer saccharum (sugar maple) Cunninghamia lanceolata (cunninghamia) Betula pendula -

Conservation Trees and Shrubs for Montana
Conservation Trees and Shrubs for Montana Montana mt.nrcs.usda.gov Introduction When you are contemplating which tree or shrub species to plant, your first thought might be, “Will this plant thrive here?” You will want to know if the plant will tolerate the temperatures, moisture, and soil conditions of the area. This publication focuses on identifying and describing trees and shrubs capable of tolerating Montana’s severe climatic and environmental conditions, the site conditions where they are best adapted to grow, and some of the benefits each tree and shrub provides. When looking at each of the provided attributes, consider these two points. First, these characteristics and traits are approximations, and variability within a species is quite common. Second, plant performance varies over time as a plant grows and matures. For example, even “drought tolerant” species require adequate moisture until their root systems become well established. Landowners and managers, homeowners, and others plant trees and shrubs for many reasons, including: windbreaks for livestock protection and crop production, shelterbelts for homes and farmsteads to reduce wind speed and conserve energy usage, living snow fences to trap and manage snow, hedgerows as visual and noise screens, landscaping for beautification around homes and parks, wildlife habitat and food, blossoms for pollinators such as bees, streamside and wetland restoration, reforestation following timber harvest or wildfire, and fruit and berries for human use to name just a few. Montana encompasses 93.3 million acres with temperature extremes ranging from -50 degrees F in northeast Montana, to 110 degrees F in summer in southcentral Montana. -

CONTAINER WILLOWS in the Southwestern US Using Seeds
(Taken from Native Plant Journal, vol. 4 | no. 2 | fall 2003) PROPAGATION PROTOCOL FOR CONTAINER WILLOWS In the Southwestern US using Seeds David R Dreesen | KEY WORDS Salix arizonica, S. bebbiana, The genetic and sexual diversity provided by seed S. exigua, S. irrorata, S. propagation of Salix L. (Salicaceae) species is an important scouleriana, arizona willow, consideration when planning riparian restoration projects. A bebb's willow, coyote willow, standard seed propagation protocol has been successful with bluestern willow, Scouler willow, a number of Salix species from montane habitats as well as height growth, stem diameter low elevation floodplains. Despite the very small seed size, growth, caliper, Salicaceae, most Salix are extremely rapid growers, which helps negate controlled release fertilizer some of the advantage typically attributed to cutting NOMENCLATURE ITIS (2002) propagation. Perhaps the largest hurdle to seedling propagation of Salix is the collection of seeds in the wild. However, rapid development of nursery seed orchards is possible using reproductive cuttings representing a mix of male and female plants or seedling stock which rapidly becomes reproductive for many species. Two principal differences between Salix and Populus L. (Salicaceae) influence seedling propagation strategies if nursery seed stock plants are desired. The first relates to the location of juvenile and mature wood in the 2 genera and where cuttings are usually taken. Often, Populus cuttings are juvenile because they are taken from stems close to the root crown, not from mature stems in the upper canopy. Therefore, cuttings are unlikely to have preformed floral buds that can be forced in the current year. -

Introduction to Biogeography and Tropical Biology
Alexey Shipunov Introduction to Biogeography and Tropical Biology Draft, version April 10, 2019 Shipunov, Alexey. Introduction to Biogeography and Tropical Biology. This at the mo- ment serves as a reference to major plants and animals groups (taxonomy) and de- scriptive biogeography (“what is where”), emphasizing tropics. April 10, 2019 version (draft). 101 pp. Title page image: Northern Great Plains, North America. Elaeagnus commutata (sil- verberry, Elaeagnaceae, Rosales) is in front. This work is dedicated to public domain. Contents What 6 Chapter 1. Diversity maps ............................. 7 Diversity atlas .................................. 16 Chapter 2. Vegetabilia ............................... 46 Bryophyta ..................................... 46 Pteridophyta ................................... 46 Spermatophyta .................................. 46 Chapter 3. Animalia ................................ 47 Arthropoda .................................... 47 Mollusca ..................................... 47 Chordata ..................................... 47 When 48 Chapter 4. The Really Short History of Life . 49 Origin of Life ................................... 51 Prokaryotic World ................................ 52 The Rise of Nonskeletal Fauna ......................... 53 Filling Marine Ecosystems ............................ 54 First Life on Land ................................. 56 Coal and Mud Forests .............................. 58 Pangea and Great Extinction .......................... 60 Renovation of the Terrestrial -

Guide to the Willows of Shoshone National Forest
United States Department of Agriculture Guide to the Willows Forest Service Rocky Mountain Research Station of Shoshone National General Technical Report RMRS-GTR-83 Forest October 2001 Walter Fertig Stuart Markow Natural Resources Conservation Service Cody Conservation District Abstract Fertig, Walter; Markow, Stuart. 2001. Guide to the willows of Shoshone National Forest. Gen. Tech. Rep. RMRS-GTR-83. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 79 p. Correct identification of willow species is an important part of land management. This guide describes the 29 willows that are known to occur on the Shoshone National Forest, Wyoming. Keys to pistillate catkins and leaf morphology are included with illustrations and plant descriptions. Key words: Salix, willows, Shoshone National Forest, identification The Authors Walter Fertig has been Heritage Botanist with the University of Wyoming’s Natural Diversity Database (WYNDD) since 1992. He has conducted rare plant surveys and natural areas inventories throughout Wyoming, with an emphasis on the desert basins of southwest Wyoming and the montane and alpine regions of the Wind River and Absaroka ranges. Fertig is the author of the Wyoming Rare Plant Field Guide, and has written over 100 technical reports on rare plants of the State. Stuart Markow received his Masters Degree in botany from the University of Wyoming in 1993 for his floristic survey of the Targhee National Forest in Idaho and Wyoming. He is currently a Botanical Consultant with a research emphasis on the montane flora of the Greater Yellowstone area and the taxonomy of grasses. Acknowledgments Sincere thanks are extended to Kent Houston and Dave Henry of the Shoshone National Forest for providing Forest Service funding for this project. -

Vegetation Community Monitoring at Ocmulgee National Monument, 2011
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science Vegetation Community Monitoring at Ocmulgee National Monument, 2011 Natural Resource Data Series NPS/SECN/NRDS—2014/702 ON THE COVER Duck potato (Sagittaria latifolia) at Ocmulgee National Monument. Photograph by: Sarah C. Heath, SECN Botanist. Vegetation Community Monitoring at Ocmulgee National Monument, 2011 Natural Resource Data Series NPS/SECN/NRDS—2014/702 Sarah Corbett Heath1 Michael W. Byrne2 1USDI National Park Service Southeast Coast Inventory and Monitoring Network Cumberland Island National Seashore 101 Wheeler Street Saint Marys, Georgia 31558 2USDI National Park Service Southeast Coast Inventory and Monitoring Network 135 Phoenix Road Athens, Georgia 30605 September 2014 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Data Series is intended for the timely release of basic data sets and data summaries. Care has been taken to assure accuracy of raw data values, but a thorough analysis and interpretation of the data has not been completed. Consequently, the initial analyses of data in this report are provisional and subject to change. All manuscripts in the series receive the appropriate level of peer review to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and designed and published in a professional manner. -
Whatcom County's Approved Plant List
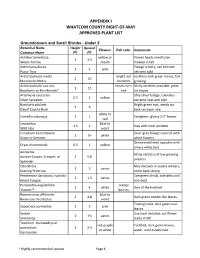
APPENDIX I WHATCOM COUNTY RIGHT-OF-WAY APPROVED PLANT LIST Groundcovers and Small Shrubs - Under 2' Botanical Name Height Spread Flowers Fall color Comments Common Name (ft) (ft) Achillea tomentosa yellow or Flower heads need to be 1 3-5 Wooly Yarrow cream mowed in fall Antennaria dioica Foliage is furry, can tolerate 1 2 pink Pussy Toes extreme cold Arctostaphylos media bright red Leathery dark green leaves, fast 2 10 Manzanita Media branches growing Arctostaphylos uva ursi leaves turn Many varieties available, great 1 15 Bearberry or Kinnikinnick* red for slopes Artemesia caucasica Silky silver foliage, tolerates 0.5 2 yellow Silver Spreader extreme heat and cold Baccharis pilularis Bright green mat, needs cut 2 6 Dwarf Coyote Bush back once per year white to Camellia sasanqua 1 1 Evergreen, glossy 2-3" leaves red Ceanothus blue to 1.5 2 Stay with local varieties Wild Lilac violet Cerastium tomentosum Silver gray foliage covered with 1 5+ white Snow-in-Summer white flowers Ornamental seed capsules with Dryas drummondii 0.5 1 yellow silvery white tails Juniperus Many varieties of low growing Juniper-Carpet, Creeper, or 2 5-8 junipers Spreader Oenothera May die back in severe winters, 1 2 varies Evening Primrose come back strong Penstemon davidsonii, rupicola Evergreen shrub, tolerates cold 2 1.5 varies Beard Tongue and heat Pyracantha augustifolia orange 1 4 white One of the hardiest 'Gnome'* berries Rosmariunus officinalis blue to 2 4-8 Dark green needle-like leaves Rosemary 'Prostratus' violet Trailing habit, dark green oval Saponaria ocymoides 1 3 pink leaves Sedum Use local varieties, cut flower 2 1-5 varies Stonecrop stalks in fall Teucrium chamaedrys or red-purple Toothed, dark green leaves, postratium 1 2-3 or white water until established Germander * Highly-recommended species Page 1 Thymus white to 2 1-2 Many varieties Thyme purple Shrubs Botanical Name Height Spread Flowers Fall color Comments Common Name (ft) (ft) Arctostaphylos bright red Shiny dark green leaves turn 2-15 20 varies Manzanita berries maroon in fall. -

Newlander Thesis FINAL
ABSTRACT In many Mojave Desert ecosystems, water infiltrates to root-zones in greatest proportion via washes. As such, washes have a pronounced effect on plant dispersion and size across these landscapes. Desert roads alter the natural spatial patterns of washes on alluvial fans (locally called bajadas) and potentially affect plant production and distribution. As a winter-rainfall dominated ecosystem, climate changes in the Mojave Desert that increase summer precipitation may also play an important role in altering vegetation processes influenced by washes. Road effects on the spatial distribution of desert plants on a Mojave Desert bajada were examined using remotely sensed LiDAR data and ground based measurements of plant size. Plant physiological responses to summer wash flow were also quantified by measuring gas exchange and water status of two dominant perennial species, Larrea tridentata and Ambrosia dumosa. Larrea and Ambrosia plants were nearly 7x and 4x larger where wash flow has been enhanced by road culverts, relative to undisturbed areas and areas where flow has been cut-off by the presence of a road/railroad. Clustering of large plants occurred along wash margins, with clustering most pronounced in areas of enhanced wash flow. No clustering was found where wash flow has been eliminated. For ecophysiological traits, both species showed pronounced responses to the pulse of water; however, these responses varied as a function of distance from wash. Larrea plants within 3 m and Ambrosia plants within ca. 2 m from the wash responded to the pulse of water. Leaf phenology dictated the timing ii of carbon gain as Larrea experienced a rapid but short-lived increase in stomatal conductance compared to a significant response for over a month following the pulse for Ambrosia. -

Checklist Flora of the Former Carden Township, City of Kawartha Lakes, on 2016
Hairy Beardtongue (Penstemon hirsutus) Checklist Flora of the Former Carden Township, City of Kawartha Lakes, ON 2016 Compiled by Dale Leadbeater and Anne Barbour © 2016 Leadbeater and Barbour All Rights reserved. No part of this publication may be reproduced, stored in a retrieval system or database, or transmitted in any form or by any means, including photocopying, without written permission of the authors. Produced with financial assistance from The Couchiching Conservancy. The City of Kawartha Lakes Flora Project is sponsored by the Kawartha Field Naturalists based in Fenelon Falls, Ontario. In 2008, information about plants in CKL was scattered and scarce. At the urging of Michael Oldham, Biologist at the Natural Heritage Information Centre at the Ontario Ministry of Natural Resources and Forestry, Dale Leadbeater and Anne Barbour formed a committee with goals to: • Generate a list of species found in CKL and their distribution, vouchered by specimens to be housed at the Royal Ontario Museum in Toronto, making them available for future study by the scientific community; • Improve understanding of natural heritage systems in the CKL; • Provide insight into changes in the local plant communities as a result of pressures from introduced species, climate change and population growth; and, • Publish the findings of the project . Over eight years, more than 200 volunteers and landowners collected almost 2000 voucher specimens, with the permission of landowners. Over 10,000 observations and literature records have been databased. The project has documented 150 new species of which 60 are introduced, 90 are native and one species that had never been reported in Ontario to date.