Using Nitrogen and Boron Isotopes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Madeline Rüegg the Patient Griselda Myth
Madeline Rüegg The Patient Griselda Myth Unauthenticated Download Date | 6/17/19 6:41 PM Unauthenticated Download Date | 6/17/19 6:41 PM Madeline Rüegg The Patient Griselda Myth Looking at Late Medieval and Early Modern European Literature Unauthenticated Download Date | 6/17/19 6:41 PM D 188 This book is published in cooperation with the project DramaNet, funded by the European Research Council ISBN 978-3-11-062870-8 e-ISBN (PDF) 978-3-11-062871-5 e-ISBN (EPUB) 978-3-11-062882-1 This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. For details go to http://creativecommons.org/licenses/by-nc-nd/4.0/. Library of Congress Control Number: 2019933417 Bibliographic information published by the Deutsche Nationalbibliothek The Deutsche Nationalbibliothek lists this publication in the Deutsche Nationalbibliografie; detailed bibliographic data are available on the Internet at http://dnb.dnb.de. © 2019 Madeline Rüegg, published by Walter de Gruyter GmbH, Berlin/Boston. This book is published with open access at www.degruyter.com. Cover image: photodeedooo/iStock/Thinkstock Typesetting: Integra Software Services Pvt. Ltd. Printing and binding: CPI books GmbH, Leck www.degruyter.com Unauthenticated Download Date | 6/17/19 6:41 PM Acknowledgments Griselda and her myth have constantly bewildered me ever since I first came across her story as an undergrad student attending Dr Katrin Rupp’s seminar on medieval tales of love at Neuchâtel University. My thoughts on the matter later grew thanks to Prof. Lukas Erne and Prof. Elisabeth Dutton, who have both helped me deepen my knowledge of the different English versions. -

Linea Mondovi'-Bastia
LINEA MONDOVI’-BASTIA Il tratto fa parte della linea Bastia-Cuneo. La tratta Cuneo-Mondovì è ancora attiva. Lunghezza linea : circa Km. 9.00 Situazione della linea: esiste ancora il binario ma si presenta in cattivo stato data l’orografia della zona attraversata. Per un futuro riutilizzo si dovrà considerare la difficoltà derivante dalla presenza delle gallerie. Comuni intercettati : Mondovì (CN) Bastia (CN) Opere d’arte principali: sono presenti due ponti in muratura a due archi sul fiume Ellero oltre a ponticelli con diversa tipologia strutturale e tre gallerie, due nel comune di Mondovì (Lmax= 244 m) oltre a vari tombini ed opere minori Criticità : non sono pertinenti i mappali n. 353, 341, 366 del foglio 15 per un totale di € 10.219,62. Sono stati demoliti i caselli al Km 5+545, al Km 3+880. LA STORIA Già intorno al 1850 erano stati costituiti i comitati promotori della costruzione di una linea ferroviaria da Cuneo a Bastia, passando per Mondovì. Quest'ultima era stata ripetutamente richiesta dal Consiglio Provinciale di Cuneo disposto a dare il suo contributo unitamente ai comuni interessati, ma non riuscirono a realizzarsi le condizioni economiche imposte dal Ministero dei Lavori Pubblici. Si dovrà attendere il Regio Decreto del 20 agosto 1873 che approvò la concessione al Comune di Mondovì per la costruzione e l'esercizio del solo tratto di ferrovia Mondovì-Bastia Mondovì. La suddetta concessione si riferiva ad un progetto di massima redatto nel 1872 dall'Ing. Soldati che prevedeva l'ubicazione della stazione terminale all'innesto della linea con la Savona-Ceva-Bra, presso Bastia Mondovì. -

SUGGESTIONS for POSSIBLE SHORT TRIPS to Get An
SUGGESTIONS FOR POSSIBLE SHORT TRIPS To get an introduction about the area near the conference: Why You Really Should Visit Italy's Outstanding Barolo Wine Region https://www.forbes.com/sites/catherinesabino/2018/10/28/why-you-really-should-visit-italys- outstanding-barolo-wine-region/#7bd3de632f89 Note that the Langa district is best visited by car. Because of its territory, the district is not accessible by train (except for Alba, which is connected to the railway system). However, driving in the Langa district should not be a problem since it is in the countryside and not in a big city. For reference, Bra and Alba have ~30,000 people each. Some of the villages referenced below are much smaller: Pollenzo (a subdivision of Bra) and Barolo have only ~700 people. Furthermore, besides the picturesque hills and the historic castles and buildings, this area is also about food and wine. So you can pair some of the suggestions below with the restaurant suggestions also available on the conference website. There are also several wine tours that are normally organized in the area and, besides the suggestions below, you can just search online for what suits your interest. 1) Tour of the Barolo Langa district: Barolo, La Morra, and Verduno Note: Requires a car. Also, distances are short and so one can break these itineraries into smaller ones as desired. Barolo (https://en.wikipedia.org/wiki/Barolo,_Piedmont) is the town in the middle of the vineyards of nebbiolo grapes that produce the famous wine of the same name. It is a medieval village developed at the foot of the castle. -

(Cn) Centro Diurno Per Anziani Alba
Denominazione Comune Indirizzo CASA DI RIPOSO A.B. OTTOLENGHI ALBA CORSO ASTI 3, 12051 ALBA (CN) CENTRO DIURNO PER ANZIANI ALBA VIA GENERAL GOVONE 11, 12051 ALBA (CN) CENTRO RIABILITAZIONE FERRERO ALBA VIA DE AMICIS 16, 12051 ALBA (CN) COMUNITA' FAMILIARE C/O CASA DI RIPOSO A.B. OTTOLENGHI ALBA CORSO ASTI 3, 12051 ALBA (CN) PICCOLA CASA DELLA DIVINA PROVVIDENZA - COTTOLENGO DI TORINO ALBA VIA VERNAZZA 10, 12051 ALBA (CN) RESIDENZA LA LUNA BAGNASCO VIA NAZIONALE 59, 12071 BAGNASCO (CN) IPAB D. BERTONE BAGNOLO PIEMONTE C.SO VITTORIO EMANUELE 32, 12031 BAGNOLO PIEMONTE (CN) FONDAZIONE CASA DI RIPOSO DON ERNESTO UBERTI BARGE VIA GALLO 11, 12032 BARGE (CN) ISTITUTO S. DOMENICO BARGE VIA COTTOLENGO 3, 12032 BARGE (CN) RESIDENZA LE RONDINI BATTIFOLLO VIA CANTONE 8/A, 12070 BATTIFOLLO (CN) CASA DI RIPOSO CASA NOSTRA BEINETTE VIA ROMA 27, 12081 BEINETTE (CN) RSA PAOLA GAMBARA BENE VAGIENNA VIA DELL'OSPEDALE 5 , 12041 BENE VAGIENNA (CN) CASA DON DALMASSO BERNEZZO VIA VILLANIS 16, 12010 BERNEZZO (CN) RSA PADRE FANTINO BORGO SAN DALMAZZO VIA MONTE BIANCO 19, 12011 BORGO SAN DALMAZZO (CN) CASA SPERANZA BOVES VIA FUNGA 79, 12012 BOVES (CN) OPERE ASSISTENZIALI UNIFICATE MONS. CALANDRI BOVES PIAZZA SAN GIOVANNI BOSCO 1, 12012 BOVES (CN) SOGGIORNO AURORA BOVES VIA CHIESA VECCHIA 5, 12012 BOVES (CN) STELLA DEL MATTINO BOVES VIA MELLANA 9, 12012 BOVES (CN) I GLICINI BRA VIA S.GIOVANNI LONTANO 31, 12042 BRA (CN) PICCOLA CASA DELLA DIVINA PROVVIDENZA - COTTOLENGO DI TORINO BRA VIA FRATELLI CARANDO 28, 12042 BRA (CN) RESIDENZA MARIO FRANCONE BRA VIA UMBERTO I 29, 12042 BRA (CN) RESIDENZA MONTEPULCIANO BRA STRADA MONTEPULCIANO 76, 12042 BRA (CN) SOGGIORNO L'IMMACOLATA BRA VIA VISCONTI VENOSTA 82 - FRAZ. -

Determina 22-17
COPIA COMUNE DI SOMMARIVA PERNO PROVINCIA DI CUNEO Servizio Tecnico Area Tecnico Manutentiva – Lavori Pubblici Determina N. 22 in data 4/04/2017 OGGETTO : NOMINA COMMISSIONE GIUDICATRICE AFFIDAMENTO SERVIZI TECNICI DI INGEGNERIA ED ARCHITETTURA PER ESECUZIONE DEI LAVORI DI DEMOLIZIONE PARTE DELLA SEDE SCOLASTICA ED AMPLIAMENTO DEL CORPO DI FABBRICA PRINCIPALE ALL’ESTERNO DELLA SAGOMA ESITENTE (CIG 6362789396 – CUP D27B15000250001) Ai sensi dell’art.4 del Regolamento Comunale sui controlli interni, approvato con delibera del Consiglio Comunale n.5 del 21/02/2013, si attesta la regolarità e la correttezza dell’azione amministrativa ed il rispetto delle norme inerenti l’emanazione del presente atto. Il Responsabile del Procedimento Il Responsabile del Servizio F.to Ing. Solange Pennazio F.to Dott.Torasso Simone La presente determina viene pubblicata all’albo pretorio on line per 15 giorni consecutivi ai sensi di legge. Dal 8/04/2017 al Il Segretario Comunale F.to Dott. Mennella Ciro IL RESPONSABILE DEL SERVIZIO Richiamata la determina a contrarre n. 15 del 07/03/2017 con cui, per le motivazioni ivi contenute, si stabiliva di affidare i servizi tecnici di ingegneria ed architettura per l’esecuzione dei lavori mediante procedura negoziata previa indagine di mercato come stabilito dal D.Lgs. 50/2016, ed in particolare dall’art. 36, comma 2, lettera b. Visto l’avviso pubblico di manifestazione d’interesse prot. 1013 del 08.03.2017 pubblicato sul sito del Comune di Sommariva Perno a far data dal 08/03/2017 nelle sezioni bandi e avvisi, Determine e Amministrazione trasparente. Verificato che entro il termine fissato (ore 12,00 del 23 marzo 2017) sono pervenute n. -

STATUS PROGETTO FWA Aggiornato Il 4 Novembre 2019 Da Infratel Italia
STATUS PROGETTO FWA aggiornato il 4 novembre 2019 da Infratel Italia Regione Provincia Comune STATUS PROGETTO FWA al 04 Novembre 2019 Piemonte Cuneo Acceglio Approvato progetto definitivo Piemonte Cuneo Aisone Approvato progetto definitivo Piemonte Cuneo Alba Approvato progetto definitivo Piemonte Cuneo Albaretto della Torre Approvato progetto definitivo Piemonte Cuneo Alto Approvato progetto definitivo Piemonte Cuneo Argentera In progettazione definitiva Piemonte Cuneo Arguello In approvazione progetto definitivo Piemonte Cuneo Bagnasco Approvato progetto definitivo Piemonte Cuneo Bagnolo Piemonte Approvato progetto definitivo Piemonte Cuneo Baldissero d'Alba Approvato progetto definitivo Piemonte Cuneo Barbaresco Approvato progetto definitivo Piemonte Cuneo Barge In esecuzione Piemonte Cuneo Barolo Approvato progetto definitivo Piemonte Cuneo Bastia Mondovì Approvato progetto definitivo Piemonte Cuneo Battifollo Approvato progetto definitivo Piemonte Cuneo Beinette Approvato progetto definitivo Piemonte Cuneo Bellino In esecuzione Piemonte Cuneo Belvedere Langhe Approvato progetto definitivo Piemonte Cuneo Bene Vagienna Approvato progetto definitivo Piemonte Cuneo Benevello Approvato progetto definitivo Piemonte Cuneo Bergolo In progettazione definitiva Piemonte Cuneo Bernezzo Approvato progetto definitivo Piemonte Cuneo Bonvicino In progettazione definitiva Piemonte Cuneo Borgo San Dalmazzo Lavori Completati Piemonte Cuneo Borgomale In approvazione progetto definitivo Piemonte Cuneo Bosia In progettazione definitiva Piemonte Cuneo -
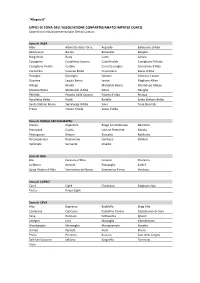
Zone Del Sistema Confartigianato Cuneo -> Comuni
“Allegato B” UFFICI DI ZONA DELL’ASSOCIAZIONE CONFARTIGIANATO IMPRESE CUNEO Zone e loro limitazione territoriale. Elenco Comuni. Zona di ALBA Alba Albaretto della Torre Arguello Baldissero d’Alba Barbaresco Barolo Benevello Bergolo Borgomale Bosia Camo Canale Castagnito Castelletto Uzzone Castellinaldo Castiglione Falletto Castiglione Tinella Castino Cerretto Langhe Corneliano d’Alba Cortemilia Cossano Belbo Cravanzana Diano d’Alba Feisoglio Gorzegno Govone Grinzane Cavour Guarene Lequio Berria Levice Magliano Alfieri Mango Montà Montaldo Roero Montelupo Albese Monteu Roero Monticello d’Alba Neive Neviglie Perletto Pezzolo Valle Uzzone Piobesi d’Alba Priocca Rocchetta Belbo Roddi Rodello Santo Stefano Belbo Santo Stefano Roero Serralunga d’Alba Sinio Tone Bormida Treiso Trezzo Tinella Vezza d’Alba Zona di BORGO SAN DALMAZZO Aisone Argentera Borgo San Dalmazzo Demonte Entracque Gaiola Limone Piemonte Moiola Pietraporzio Rittana Roaschia Robilante Roccasparvera Roccavione Sambuco Valdieri Valloriate Vernante Vinadio Zona di BRA Bra Ceresole d’Alba Cervere Cherasco La Morra Narzole Pocapaglia Sanfrè Santa Vittoria d’Alba Sommariva del Bosco Sommariva Perno Verduno Zona di CARRÙ Carrù Cigliè Clavesana Magliano Alpi Piozzo Rocca Cigliè Zona di CEVA Alto Bagnasco Battifollo Briga Alta Camerana Caprauna Castellino Tanaro Castelnuovo di Ceva Ceva Garessio Gottasecca Igliano Lesegno Lisio Marsaglia Mombarcaro Mombasiglio Monesiglio Montezemolo Nucetto Ormea Paroldo Perlo Priero Priola Prunetto Roascio Sale delle Langhe Sale San Giovanni Saliceto -

Fiera Del Porro Di Cervere - 42^ Edizione Qualifica Manifestazione: Locale Tipologia: Mostra Mercato Periodo Svolgimento: 06/11/2021 21/11/2021 Settori: Agricoltura
Comune: Cervere Provincia: Cuneo Titolo manifestazione: Fiera del Porro di Cervere - 42^ edizione Qualifica manifestazione: Locale Tipologia: Mostra mercato Periodo svolgimento: 06/11/2021 21/11/2021 Settori: Agricoltura Ente organizzatore: Comune Indirizzo manifestazione: Piazza San Sebastiano - Via IV Novembre - Referenti e numeri utili: Tel. 0172/471.000 – Fax 0172/474.833 - E-mail: [email protected] Riferimenti utili: - Sito internet: http://www.comune.cervere.cn.it - Orario apertura/chiusura: dalle 08.00 alle 23.00 - Costo ingresso: gratuito Consolidare un turismo gastronomico sviluppatosi parallelamente all’affermazione del porro, prodotto tipico locale, inserito nell’elenco dei prodotti agroalimentari tradizionali del Piemonte, è l’obiettivo della manifestazione che si propone di estendere l’interesse suscitato intorno a questo ortaggio, a tutto il comparto turistico locale. Due settimane di spettacoli, divertimenti, mercati, momenti gastronomici, all’insegna del prodotto tipico che ha reso rinomato il paese. La tradizionale Fiera, nata per creare attenzione intorno ad una varietà di porro che da più di vent’anni rischiava l’estinzione è incentrata sulla degustazione di piatti che hanno come ingrediente principale il prelibato ortaggio. Per sottolineare il senso di appartenenza al territorio Piemontese, è stato ritenuto opportuno dall’Amministrazione Comunale, estendere le degustazioni anche ad altri prodotti tipici della gastronomia locale come il riso e la salsiccia di Bra abbinati entrambi ai Porri di Cervere ed ai migliori vini del Piemonte. La manifestazione, che ha assunto i connotati di un vero e Presentazione sintetica proprio evento, ogni anno nella seconda e terza settimana di novembre, della manifestazione raduna migliaia di visitatori che, oltre ad affollare lo stand della rassegna gastronomica, raggiungono forti presenze numeriche nei ristoranti locali ed in quelli dei Comuni vicini. -

Orari E Percorsi Della Linea Treno R
Orari e mappe della linea treno R Ceva Visualizza In Una Pagina Web La linea treno R (Ceva) ha 9 percorsi. Durante la settimana è operativa: (1) Ceva: 18:45 - 22:17 (2) Cuneo: 05:25 - 23:25 (3) Fossano: 05:02 - 20:31 (4) San Giuseppe Di Cairo: 08:22 - 18:22 (5) Savona: 06:22 - 20:25 (6) Torino P.Nuova: 04:21 - 21:24 (7) Ventimiglia: 06:25 - 14:25 Usa Moovit per trovare le fermate della linea treno R più vicine a te e scoprire quando passerà il prossimo mezzo della linea treno R Direzione: Ceva Orari della linea treno R 12 fermate Orari di partenza verso Ceva: VISUALIZZA GLI ORARI DELLA LINEA lunedì 18:45 - 22:17 martedì 18:45 - 22:17 Torino Porta Nuova 2a Via Nizza, Torino mercoledì 18:45 - 22:17 Torino Lingotto giovedì 18:45 - 22:17 1 Via Mario Pannunzio, Torino venerdì 18:45 - 22:17 Carmagnola sabato 19:55 - 22:17 7 Piazza Xxx Aprile, Carmagnola domenica 22:17 Racconigi 1 Piazza Giuseppe Mazzini, Racconigi Savigliano 1 Piazza Casimiro Sperino, Savigliano Informazioni sulla linea treno R Direzione: Ceva Fossano Fermate: 12 5 Piazza Kennedy, Fossano Durata del tragitto: 32 min La linea in sintesi: Torino Porta Nuova, Torino Trinità-Bene Vagienna Lingotto, Carmagnola, Racconigi, Savigliano, 50 Via Stazione, Trinità Fossano, Trinità-Bene Vagienna, Magliano-Crava- Morozzo, Mondovì, Vicoforte-San Michele, Lesegno, Magliano-Crava-Morozzo Ceva Mondovì 2 Piazza Centro Franco, Mondovì Vicoforte-San Michele Lesegno Ceva 6 Piazzale Stazione, Ceva Direzione: Cuneo Orari della linea treno R 12 fermate Orari di partenza verso Cuneo: VISUALIZZA GLI -

Medie Radon Provincia Cuneo 2017
Provincia Comune media radon al piano terra (Bq/m 3) Cuneo Acceglio 133 Cuneo Aisone 149 Cuneo Alba 99 Cuneo Albaretto della torre 79 Cuneo Alto 498 Cuneo Argentera 216 Cuneo Arguello 79 Cuneo Bagnasco 112 Cuneo Bagnolo Piemonte 135 Cuneo Baldissero d'Alba 105 Cuneo Barbaresco 89 Cuneo Barge 145 Cuneo Barolo 85 Cuneo Bastia mondovi' 108 Cuneo Battifollo 96 Cuneo Beinette 160 Cuneo Bellino 80 Cuneo Belvedere Langhe 79 Cuneo Bene Vagienna 148 Cuneo Benevello 79 Cuneo Bergolo 81 Cuneo Bernezzo 102 Cuneo Bonvicino 79 Cuneo Borgo San Dalmazzo 133 Cuneo Borgomale 79 Cuneo Bosia 87 Cuneo Bossolasco 79 Cuneo Boves 140 Cuneo Bra 146 Cuneo Briaglia 82 Cuneo Briga Alta 125 Cuneo Brondello 120 Cuneo Brossasco 118 Cuneo Busca 148 Cuneo Camerana 83 Cuneo Camo 80 Cuneo Canale 107 Cuneo Canosio 130 Cuneo Caprauna 602 Cuneo Caraglio 63 Cuneo Caramagna Piemonte 157 Cuneo Carde' 155 Cuneo Carru' 147 Cuneo Cartignano 116 Cuneo Casalgrasso 154 Cuneo Castagnito 92 Cuneo Casteldelfino 90 Cuneo Castellar 143 Cuneo Castelletto Stura 154 Cuneo Castelletto Uzzone 81 Cuneo Castellinaldo 98 Cuneo Castellino Tanaro 85 Cuneo Castelmagno 96 Cuneo Castelnuovo di Ceva 99 Cuneo Castiglione Falletto 94 Cuneo Castiglione Tinella 81 Cuneo Castino 81 Cuneo Cavallerleone 161 Cuneo Cavallermaggiore 160 Cuneo Celle di Macra 73 Cuneo Centallo 159 Cuneo Ceresole d'Alba 151 Cuneo Cerretto Langhe 79 Cuneo Cervasca 142 Cuneo Cervere 151 Cuneo Ceva 105 Cuneo Cherasco 140 Cuneo Chiusa di Pesio 147 Cuneo Ciglie' 98 Cuneo Cissone 79 Cuneo Clavesana 94 Cuneo Corneliano d'Alba 104 Cuneo -

CUNEO E Provincia INFORMASALUTE Accesso Al Servizio Sanitario Nazionale Per I Cittadini Non Comunitari
Progetto cofinanziato da ISTITUTO NAZIONALE UNIONE SALUTE, MIGRAZIONI MINISTERO MINISTERO EUROPEA E POVERTÀ DELLA SALUTE DELL’INTERNO Fondo Europeo per l'Integrazione dei cittadini di Paesi terzi INFORMASALUTE Accesso al Servizio Sanitario Nazionale per i cittadini non comunitari I Servizi Sanitari di CUNEO e Provincia INFORMASALUTE Accesso al Servizio Sanitario Nazionale per i cittadini non comunitari I PRINCIPALI SERVIZI SANITARI DI CUNEO E PROVINCIA I PRINCIPALI SERVIZI SANITARI DI CUNEO E PROVINCIA I PRINCIPALI SERVIZI SANITARI DI CUNEO E PROVINCIA Il territorio provinciale di Cuneo è diviso in due ASL (Aziende Sanitarie Locali) ognuna delle quali ha una sede principale e una serie di distretti socio-sanitari diffusi in modo da facilitare l’ac- cesso dei cittadini: • ASL CN1 divisa in 6 distretti • ASL CN2 divisa in 2 distretti • TERRITORI DI RIFERIMENTO 2 E UFFICI RELAZIONI CON IL PUBBLICO (URP) ASL CN1 Tel. 0171.450221 · Via C. Boggio 12 DISTRETTO CUNEO - BORGO SAN DALMAZZO Aisone, Argentera, Beinette, Borgo San Dalmazzo, Boves, Castelletto Stura, Centallo, Chiusa di Pesio, Cuneo, Demonte, Entracque, Gaiola, Limone Piemonte, Margarita, Moiola, Montanera, Morozzo, Peveragno, Pietraporzio, Rittana, Roaschia, Robilante, Roccasparvera, Roccavione, Sambuco, Tarantasca, Valdieri, Valloriate, Vernante, Vignolo, Vinadio Via C. Boggio 12 - Tel. 0171.450221 · Da lunedì a venerdì 8.30-12.30 DISTRETTO DRONERO Acceglio, Bernezzo, Busca, Canosio, Caraglio, Cartignano, Castelmagno, Celle Di Macra, Cervasca, Dronero, Elva, TERRITORI DI RIFERIMENTO -

Modello Sedi Disponibili CN PRIMARIA
SCUOLA PRIMARIA Codice meccanografico Codice meccanografico Denominazione Comune Tipologia di posto Istituto principale Sede CNIC85600V CNEE856011 ALBA CENTRO STORICO ALBA COMUNE CNIC85600V CNEE856011 ALBA CENTRO STORICO ALBA COMUNE CNIC85600V CNEE856011 ALBA CENTRO STORICO ALBA COMUNE CNIC855003 CNEE855015ALBA ALBA QUARTIERE MORETTA ALBA COMUNE CNIC855003 CNEE855015ALBA ALBA QUARTIERE MORETTA ALBA COMUNE CNIC855003 CNEE855015ALBA ALBA QUARTIERE MORETTA ALBA COMUNE CNIC855003 CNEE855015ALBA ALBA QUARTIERE MORETTA ALBA COMUNE CNIC855003 CNEE855015ALBA ALBA QUARTIERE MORETTA ALBA COMUNE CNIC86400T CNEE86401X BRA 1 BRA COMUNE CNIC86400T CNEE86401X BRA 1 BRA COMUNE CNIC86400T CNEE86401X BRA 1 BRA COMUNE CNIC838009 CNEE83802C CAVALLERMAGGIORE CAVALLERMAGGIORE COMUNE CNIC838009 CNEE83802C CAVALLERMAGGIORE CAVALLERMAGGIORE COMUNE CNIC825007 CNEE825019 CHERASCO CHERASCO COMUNE CNIC825007 CNEE825019 CHERASCO CHERASCO COMUNE CNIC825007 CNEE825019 CHERASCO CHERASCO COMUNE CNIC825007 CNEE825019 CHERASCO CHERASCO COMUNE CNIC825007 CNEE825019 CHERASCO CHERASCO COMUNE CNIC825007 CNEE825019 CHERASCO CHERASCO COMUNE CNIC84900Q CNEE849052 DIANO D'ALBA DIANO D'ALBA COMUNE CNIC84900Q CNEE849052 DIANO D'ALBA DIANO D'ALBA COMUNE CNIC84900Q CNEE849052 DIANO D'ALBA DIANO D'ALBA COMUNE CNIC84900Q CNEE849052 DIANO D'ALBA DIANO D'ALBA COMUNE CNIC84900Q CNEE849052 DIANO D'ALBA DIANO D'ALBA COMUNE CNIC83600N CNEE83602R GARESSIO GARESSIO COMUNE CNIC82300G CNEE82303Q GOVONE GOVONE COMUNE CNIC81900X CNEE819034 LA MORRA LA MORRA COMUNE CNIC81900X CNEE819034 LA MORRA