Recommendations for Evaluation and Monitoring of Patients with Joubert Syndrome and Related Disorders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

American Board of Psychiatry and Neurology, Inc
AMERICAN BOARD OF PSYCHIATRY AND NEUROLOGY, INC. CERTIFICATION EXAMINATION IN NEUROLOGY 2015 Content Blueprint (January 13, 2015) Part A Basic neuroscience Number of questions: 120 01. Neuroanatomy 3-5% 02. Neuropathology 3-5% 03. Neurochemistry 2-4% 04. Neurophysiology 5-7% 05. Neuroimmunology/neuroinfectious disease 2-4% 06. Neurogenetics/molecular neurology, neuroepidemiology 2-4% 07. Neuroendocrinology 1-2% 08. Neuropharmacology 4-6% Part B Behavioral neurology, cognition, and psychiatry Number of questions: 80 01. Development through the life cycle 3-5% 02. Psychiatric and psychological principles 1-3% 03. Diagnostic procedures 1-3% 04. Clinical and therapeutic aspects of psychiatric disorders 5-7% 05. Clinical and therapeutic aspects of behavioral neurology 5-7% Part C Clinical neurology (adult and child) The clinical neurology section of the Neurology Certification Examination is comprised of 60% adult neurology questions and 40% child neurology questions. Number of questions: 200 01. Headache disorders 1-3% 02. Pain disorders 1-3% 03. Epilepsy and episodic disorders 1-3% 04. Sleep disorders 1-3% 05. Genetic disorders 1-3% 2015 ABPN Content Specifications Page 1 of 22 Posted: Certification in Neurology AMERICAN BOARD OF PSYCHIATRY AND NEUROLOGY, INC. 06. Congenital disorders 1-3% 07. Cerebrovascular disease 1-3% 08. Neuromuscular diseases 2-4% 09. Cranial nerve palsies 1-3% 10. Spinal cord diseases 1-3% 11. Movement disorders 1-3% 12. Demyelinating diseases 1-3% 13. Neuroinfectious diseases 1-3% 14. Critical care 1-3% 15. Trauma 1-3% 16. Neuro-ophthalmology 1-3% 17. Neuro-otology 1-3% 18. Neurologic complications of systemic diseases 2-4% 19. -

Joubert Syndrome Genereview
Title: Joubert Syndrome GeneReview — Molecular Genetics: Less Common Genetic Causes Authors: Parisi M, Glass I Updated: June 2017 Note: The following information is provided by the authors listed above and has not been reviewed by GeneReviews staff. Joubert Syndrome: Less Common Genetic Causes ARL13B B9D1 B9D2 CEP41 IFT172 KIF7 OFD1 (CXORF5) PDE6D POC1B TCTN1 TCTN3 TMEM138 TMEM231 TMEM237 (ALS2CR4) TTC21B ARL13B Gene structure. ARL13B is a ten-exon gene that encodes a 428-amino acid protein. Pathogenic variants. Two families with a phenotype typical of classic Joubert syndrome had missense and/or nonsense variants in this gene; one of these individuals also had evidence of a retinopathy [Cantagrel et al 2008]. Normal gene product. ARL13B encodes ADP-ribosylation factor-like protein 13B, a member of the ADP-ribosylation factor-like family. Multiple transcript variants result from alternate splicing; two protein isoforms are known. The AR13B protein is a small GTPase in the Ras superfamily that contains both N-terminal and C-terminal guanine nucleotide-binding motifs. It is localized to the cilia and plays a role in cilia formation and maintenance as well as sonic hedgehog signaling. Abnormal gene product. In C elegans, pathogenic variants in the homolog arl13 exhibit defective cilium morphology, localization, and anterograde intraflagellar transport [Cevik et al 2010]. Mice with defects in the murine ortholog have neural tube defects and polydactyly, as well as an embryonic-lethal phenotype [Cantagrel et al 2008, Doherty 2009]. B9D1. See Tables A and B. B9D2. See Tables A and B. CEP41 Gene structure. The gene consists of 11 exons and spans approximately 50 kb. -

Joubert Syndrome and Related Disorders
Brancati et al. Orphanet Journal of Rare Diseases 2010, 5:20 http://www.ojrd.com/content/5/1/20 REVIEW Open Access JoubertReview Syndrome and related disorders Francesco Brancati1,2, Bruno Dallapiccola3 and Enza Maria Valente*1,4 Abstract Joubert syndrome (JS) and related disorders (JSRD) are a group of developmental delay/multiple congenital anomalies syndromes in which the obligatory hallmark is the molar tooth sign (MTS), a complex midbrain-hindbrain malformation visible on brain imaging, first recognized in JS. Estimates of the incidence of JSRD range between 1/80,000 and 1/ 100,000 live births, although these figures may represent an underestimate. The neurological features of JSRD include hypotonia, ataxia, developmental delay, intellectual disability, abnormal eye movements, and neonatal breathing dysregulation. These may be associated with multiorgan involvement, mainly retinal dystrophy, nephronophthisis, hepatic fibrosis and polydactyly, with both inter- and intra-familial variability. JSRD are classified in six phenotypic subgroups: Pure JS; JS with ocular defect; JS with renal defect; JS with oculorenal defects; JS with hepatic defect; JS with orofaciodigital defects. With the exception of rare X-linked recessive cases, JSRD follow autosomal recessive inheritance and are genetically heterogeneous. Ten causative genes have been identified to date, all encoding for proteins of the primary cilium or the centrosome, making JSRD part of an expanding group of diseases called "ciliopathies". Mutational analysis of causative genes is available in few laboratories worldwide on a diagnostic or research basis. Differential diagnosis must consider in particular the other ciliopathies (such as nephronophthisis and Senior-Loken syndrome), distinct cerebellar and brainstem congenital defects and disorders with cerebro-oculo-renal manifestations. -

Renal Cystic Disorders Infosheet 6-14-19
Next Generation Sequencing Panel for Renal Cystic Disorders Clinical Features: Renal cystic diseases are a genetically heterogeneous group of conditions characterized By isolated renal disease or renal cysts in conjunction with extrarenal features (1). Age of onset of renal cystic disease ranges from neonatal to adult onset. Common features of renal cystic diseases include renal insufficiency and progression to end stage renal disease (ESRD). Identification of the genetic etiology of renal cystic disease can aid in appropriate clinical management of the affected patient. Our Renal Cystic Disorders Panel includes sequence and deletion/duplicaton analysis of all 79 genes listed below. Renal Cystic Disorders Sequencing Panel AHI1 BMPER HNF1B NEK8 TCTN3 WDPCP ANKS6 C5orf42 IFT27 NOTCH2 TFAP2A WDR19 ARL13B CC2D2A IFT140 NPHP1 TMEM107 XPNPEP3 ARL6 CDC73 IFT172 NPHP3 TMEM138 ZNF423 B9D1 CEP104 INPP5E NPHP4 TMEM216 B9D2 CEP120 INVS OFD1 TMEM231 BBIP1 CEP164 IQCB1 PDE6D TMEM237 BBS1 CEP290 JAG1 PKD2 TMEM67 BBS10 CEP41 KIAA0556 PKHD1 TRIM32 BBS12 CEP83 KIAA0586 REN TSC1 BBS2 CRB2 KIF14 RPGRIP1L TSC2 BBS4 CSPP1 KIF7 SALL1 TTC21B BBS5 DCDC2 LZTFL1 SDCCAG8 TTC8 BBS7 GLIS2 MKKS TCTN1 UMOD BBS9 GLIS3 MKS1 TCTN2 VHL Disorder Genes Inheritance Clinical features/molecular genetics Bardet Biedl ARL6 AR Bardet-Biedl syndrome (BBS) is an autosomal syndrome BBS1 recessive multi-systemic ciliopathy characterized By BBS10 retinal dystrophy, oBesity, postaxial polydactyly, BBS12 leaning difficulties, renal involvement and BBS2 genitourinary abnormalities (2). Visual prognosis is BBS4 poor, and the mean age of legal Blindness is 15.5 BBS5 years. Birth weight is typically normal But significant BBS7 weight gain Begins within the first year. Renal BBS9 disease is a major cause of morBidity and mortality. -

Autism Spectrum Disorders—A Genetics Review Judith H
GENETEST REVIEW Genetics in Medicine Autism spectrum disorders—A genetics review Judith H. Miles, MD, PhD TABLE OF CONTENTS Prevalence .........................................................................................................279 Adenylosuccinate lyase deficiency ............................................................285 Clinical features................................................................................................279 Creatine deficiency syndromes..................................................................285 Core autism symptoms...................................................................................279 Smith-Lemli-Opitz syndrome.....................................................................285 Diagnostic criteria and tools..........................................................................280 Other single-gene disorders.......................................................................285 Neurologic and medical symptoms .............................................................281 Developmental syndromes of undetermined etiology..............................286 Genetics of autism...........................................................................................281 Moebius syndrome or sequence...............................................................286 Chromosomal disorders and CNVS..............................................................282 Landau-Kleffner syndrome .........................................................................286 Single-gene -
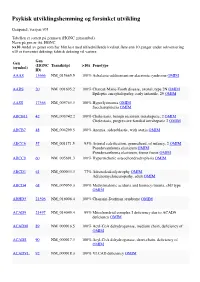
Psykisk Utviklingshemming Og Forsinket Utvikling
Psykisk utviklingshemming og forsinket utvikling Genpanel, versjon v03 Tabellen er sortert på gennavn (HGNC gensymbol) Navn på gen er iht. HGNC >x10 Andel av genet som har blitt lest med tilfredstillende kvalitet flere enn 10 ganger under sekvensering x10 er forventet dekning; faktisk dekning vil variere. Gen Gen (HGNC Transkript >10x Fenotype (symbol) ID) AAAS 13666 NM_015665.5 100% Achalasia-addisonianism-alacrimia syndrome OMIM AARS 20 NM_001605.2 100% Charcot-Marie-Tooth disease, axonal, type 2N OMIM Epileptic encephalopathy, early infantile, 29 OMIM AASS 17366 NM_005763.3 100% Hyperlysinemia OMIM Saccharopinuria OMIM ABCB11 42 NM_003742.2 100% Cholestasis, benign recurrent intrahepatic, 2 OMIM Cholestasis, progressive familial intrahepatic 2 OMIM ABCB7 48 NM_004299.5 100% Anemia, sideroblastic, with ataxia OMIM ABCC6 57 NM_001171.5 93% Arterial calcification, generalized, of infancy, 2 OMIM Pseudoxanthoma elasticum OMIM Pseudoxanthoma elasticum, forme fruste OMIM ABCC9 60 NM_005691.3 100% Hypertrichotic osteochondrodysplasia OMIM ABCD1 61 NM_000033.3 77% Adrenoleukodystrophy OMIM Adrenomyeloneuropathy, adult OMIM ABCD4 68 NM_005050.3 100% Methylmalonic aciduria and homocystinuria, cblJ type OMIM ABHD5 21396 NM_016006.4 100% Chanarin-Dorfman syndrome OMIM ACAD9 21497 NM_014049.4 99% Mitochondrial complex I deficiency due to ACAD9 deficiency OMIM ACADM 89 NM_000016.5 100% Acyl-CoA dehydrogenase, medium chain, deficiency of OMIM ACADS 90 NM_000017.3 100% Acyl-CoA dehydrogenase, short-chain, deficiency of OMIM ACADVL 92 NM_000018.3 100% VLCAD -

Blueprint Genetics Comprehensive Growth Disorders / Skeletal
Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders Panel Test code: MA4301 Is a 374 gene panel that includes assessment of non-coding variants. This panel covers the majority of the genes listed in the Nosology 2015 (PMID: 26394607) and all genes in our Malformation category that cause growth retardation, short stature or skeletal dysplasia and is therefore a powerful diagnostic tool. It is ideal for patients suspected to have a syndromic or an isolated growth disorder or a skeletal dysplasia. About Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders This panel covers a broad spectrum of diseases associated with growth retardation, short stature or skeletal dysplasia. Many of these conditions have overlapping features which can make clinical diagnosis a challenge. Genetic diagnostics is therefore the most efficient way to subtype the diseases and enable individualized treatment and management decisions. Moreover, detection of causative mutations establishes the mode of inheritance in the family which is essential for informed genetic counseling. For additional information regarding the conditions tested on this panel, please refer to the National Organization for Rare Disorders and / or GeneReviews. Availability 4 weeks Gene Set Description Genes in the Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders Panel and their clinical significance Gene Associated phenotypes Inheritance ClinVar HGMD ACAN# Spondyloepimetaphyseal dysplasia, aggrecan type, AD/AR 20 56 Spondyloepiphyseal dysplasia, Kimberley -

Blueprint Genetics Comprehensive Skeletal Dysplasias and Disorders
Comprehensive Skeletal Dysplasias and Disorders Panel Test code: MA3301 Is a 251 gene panel that includes assessment of non-coding variants. Is ideal for patients with a clinical suspicion of disorders involving the skeletal system. About Comprehensive Skeletal Dysplasias and Disorders This panel covers a broad spectrum of skeletal disorders including common and rare skeletal dysplasias (eg. achondroplasia, COL2A1 related dysplasias, diastrophic dysplasia, various types of spondylo-metaphyseal dysplasias), various ciliopathies with skeletal involvement (eg. short rib-polydactylies, asphyxiating thoracic dysplasia dysplasias and Ellis-van Creveld syndrome), various subtypes of osteogenesis imperfecta, campomelic dysplasia, slender bone dysplasias, dysplasias with multiple joint dislocations, chondrodysplasia punctata group of disorders, neonatal osteosclerotic dysplasias, osteopetrosis and related disorders, abnormal mineralization group of disorders (eg hypopohosphatasia), osteolysis group of disorders, disorders with disorganized development of skeletal components, overgrowth syndromes with skeletal involvement, craniosynostosis syndromes, dysostoses with predominant craniofacial involvement, dysostoses with predominant vertebral involvement, patellar dysostoses, brachydactylies, some disorders with limb hypoplasia-reduction defects, ectrodactyly with and without other manifestations, polydactyly-syndactyly-triphalangism group of disorders, and disorders with defects in joint formation and synostoses. Availability 4 weeks Gene Set Description -

Irish Rare Kidney Disease Network (IRKDN)
Irish Rare kidney Disease Network (IRKDN) Others Cork University Mater, Waterford University Dr Liam Plant Hospital Galway Dr Abernathy University Hospital Renal imaging Dr M Morrin Prof Griffin Temple St and Crumlin Beaumont Hospital CHILDRENS Hospital Tallaght St Vincents Dr Atiff Awann Rare Kidney Disease Clinic Hospital University Hospital Prof Peter Conlon Dr Lavin Prof Dr Holian Little Renal pathology Lab Limerick University Dr Dorman and Hospital Dr Doyle Dr Casserly Patient Renal Council Genetics St James Laboratory Hospital RCSI Dr Griffin Prof Cavaller MISION Provision of care to patients with Rare Kidney Disease based on best available medical evidence through collaboration within Ireland and Europe Making available clinical trials for rare kidney disease to Irish patients where available Collaboration with other centres in Europe treating rare kidney disease Education of Irish nephrologists on rare Kidney Disease. Ensuring a seamless transition of children from children’s hospital with rare kidney disease to adult centres with sharing of knowledge of rare paediatric kidney disease with adult centres The provision of precise molecular diagnosis of patients with rare kidney disease The provision of therapeutic plan based on understanding of molecular diagnosis where available Development of rare disease specific registries within national renal It platform ( Emed) Structure Beaumont Hospital will act as National rare Kidney Disease Coordinating centre working in conjunction with a network of Renal unit across the country -

Joubert Syndrome
Joubert syndrome Description Joubert syndrome is a disorder that affects many parts of the body. The signs and symptoms of this condition vary among affected individuals, even among members of the same family. The hallmark feature of Joubert syndrome is a combination of brain abnormalities that together are known as the molar tooth sign, which can be seen on brain imaging studies such as magnetic resonance imaging (MRI). This sign results from the abnormal development of structures near the back of the brain, including the cerebellar vermis and the brainstem. The molar tooth sign got its name because the characteristic brain abnormalities resemble the cross-section of a molar tooth when seen on an MRI. Most infants with Joubert syndrome have low muscle tone (hypotonia) in infancy, which contributes to difficulty coordinating movements (ataxia) in early childhood. Other characteristic features of the condition include episodes of unusually fast (hyperpnea) or slow (apnea) breathing in infancy, and abnormal eye movements (ocular motor apraxia). Most affected individuals have delayed development and intellectual disability, which can range from mild to severe. Distinctive facial features can also occur in Joubert syndrome; these include a broad forehead, arched eyebrows, droopy eyelids (ptosis), widely spaced eyes (hypertelorism), low-set ears, and a triangle-shaped mouth. Joubert syndrome can include a broad range of additional signs and symptoms. The condition is sometimes associated with other eye abnormalities (such as retinal dystrophy, which can cause vision loss, and coloboma, which is a gap or split in a structure of the eye), kidney disease (including polycystic kidney disease and nephronophthisis), liver disease, skeletal abnormalities (such as the presence of extra fingers and toes), or hormone (endocrine) problems. -

The CETT Program Is an Initiative Developed and Sponsored by the NIH Office of Rare Diseases
Researchers By establishing a relationship with a Clinical Laboratory Improvement Amendments (CLIA)- approved laboratory and patient advocate group, researchers can: • Focus resources on research questions, not on clinical care and service. • Provide clinical results to their research participants, patients, families and referring clinicians in an approved regulatory manner. • Identify potential new subjects from clinical testing for research participation with documented clinical findings. • Fulfill their commitment to the research community by bringing research efforts to clinical practice. • Ensure that the knowledge base obtained from the collection and storage of clinical data helps move research forward on the rare disease. Laboratory Directors By establishing relationships with researchers and patient advocates, laboratories can: • Provide quality testing and improve access to control materials. • Obtain expert help on test result interpretation. • Obtain expert help to develop appropriate educational materials for the public about the genetic test and appropriate referrals of individuals and families for genetic testing. • Expand awareness of test availability to the clinical and patient communities. • Collect and store genetic test information to increase the ability to interpret future test results. • Work with patient advocates to increase knowledge about testing and understanding the research and clinical data. Patient Advocates By establishing relationships with researchers and Clinical Laboratory Improvement Amendments (CLIA)-certified laboratories, patient advocates can: • Provide their members and their families a quality diagnostic test for the rare condition. • Increase access to all testing needs ⎯ diagnosis, carrier, prenatal and pre-implantation. • Provide appropriate resources and educational materials to increase understanding of the rare disease and the genetic test. • Develop a database of information on the genetic test that would help with future interpretation of the genetic test and clinical expression of the rare disease. -

Joubert Syndrome and Related Disorders Precision Panel Overview Indications Clinical Utility
Joubert Syndrome and Related Disorders Precision Panel Overview Joubert Syndrome (JS) and Related Disorders (JSRD) are a group of ciliopathies characterized by mid-hindbrain malformation, developmental delay, hypotonia, oculomotor apraxia, and breathing abnormalities. Cilia play a crucial role in appropriate axonal growth and connectivity which are essential for functional wiring of the brain. The classic midbrain-hindbrain malformation is a hallmark image finding known as molar tooth sign. Joubert Syndrome and Related Disorders are a group of clinically and genetically heterogeneous disorders involving ciliopathy-related genes. Therefore, clinical manifestations have multiorgan involvement, mainly retinal dystrophy, hepatic fibrosis and polydactyly, among others. With the exception of rare X-linked recessive cases, Joubert Syndrome and Related Disorders follow an autosomal recessive inheritance pattern. The Igenomix Joubert Syndrome and Related Disorders Precision Panel can serve as a screening and diagnostic tool ultimately leading to a better management and prognosis of the disease. It provides a comprehensive analysis of the genes involved in this disease using next-generation sequencing (NGS) to fully understand the spectrum of relevant genes involved. Indications The Igenomix Joubert Syndrome and Related Disorders Precision Panel is indicated for those patients with clinical and/or imaging findings suggestive of Joubert Syndrome and Related Disorders presenting with the following manifestations: ‐ Hypotonia ‐ Ataxia ‐ Developmental delay ‐ Abnormal eye and tongue movements ‐ Respiratory control disturbances ‐ Polydactyly ‐ Cleft lip or palate ‐ Seizures Clinical Utility The clinical utility of this panel is: - The genetic and molecular confirmation for an accurate clinical diagnosis of a symptomatic patient. 1 - Early initiation of treatment involving a multidisciplinary team focusing on respiratory and feeding problems in neonates and infants.