Joubert Syndrome Genereview
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Educational Paper Ciliopathies
Eur J Pediatr (2012) 171:1285–1300 DOI 10.1007/s00431-011-1553-z REVIEW Educational paper Ciliopathies Carsten Bergmann Received: 11 June 2011 /Accepted: 3 August 2011 /Published online: 7 September 2011 # The Author(s) 2011. This article is published with open access at Springerlink.com Abstract Cilia are antenna-like organelles found on the (NPHP) . Ivemark syndrome . Meckel syndrome (MKS) . surface of most cells. They transduce molecular signals Joubert syndrome (JBTS) . Bardet–Biedl syndrome (BBS) . and facilitate interactions between cells and their Alstrom syndrome . Short-rib polydactyly syndromes . environment. Ciliary dysfunction has been shown to Jeune syndrome (ATD) . Ellis-van Crefeld syndrome (EVC) . underlie a broad range of overlapping, clinically and Sensenbrenner syndrome . Primary ciliary dyskinesia genetically heterogeneous phenotypes, collectively (Kartagener syndrome) . von Hippel-Lindau (VHL) . termed ciliopathies. Literally, all organs can be affected. Tuberous sclerosis (TSC) . Oligogenic inheritance . Modifier. Frequent cilia-related manifestations are (poly)cystic Mutational load kidney disease, retinal degeneration, situs inversus, cardiac defects, polydactyly, other skeletal abnormalities, and defects of the central and peripheral nervous Introduction system, occurring either isolated or as part of syn- dromes. Characterization of ciliopathies and the decisive Defective cellular organelles such as mitochondria, perox- role of primary cilia in signal transduction and cell isomes, and lysosomes are well-known -

The Hydrolethalus Syndrome Protein HYLS-1 Regulates Formation of the Ciliary Gate
ARTICLE Received 8 Sep 2015 | Accepted 30 Jun 2016 | Published 18 Aug 2016 DOI: 10.1038/ncomms12437 OPEN The hydrolethalus syndrome protein HYLS-1 regulates formation of the ciliary gate Qing Wei1,2,*, Yingyi Zhang1,*, Clementine Schouteden3, Yuxia Zhang1, Qing Zhang1, Jinhong Dong1, Veronika Wonesch3, Kun Ling1, Alexander Dammermann3 & Jinghua Hu1,4,5 Transition fibres (TFs), together with the transition zone (TZ), are basal ciliary structures thought to be crucial for cilium biogenesis and function by acting as a ciliary gate to regulate selective protein entry and exit. Here we demonstrate that the centriolar and basal body protein HYLS-1, the C. elegans orthologue of hydrolethalus syndrome protein 1, is required for TF formation, TZ organization and ciliary gating. Loss of HYLS-1 compromises the docking and entry of intraflagellar transport (IFT) particles, ciliary gating for both membrane and soluble proteins, and axoneme assembly. Additional depletion of the TF component DYF-19 in hyls-1 mutants further exacerbates TZ anomalies and completely abrogates ciliogenesis. Our data support an important role for HYLS-1 and TFs in establishment of the ciliary gate and underline the importance of selective protein entry for cilia assembly. 1 Department of Biochemistry and Molecular Biology, Mayo Clinic, Rochester, Minnesota 55905, USA. 2 Key Laboratory of Insect Developmental and Evolutionary Biology, Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai 200032, China. 3 Max F. Perutz Laboratories, Vienna Biocenter (VBC), University of Vienna, A-1030 Vienna, Austria. 4 Division of Nephrology and Hypertension, Mayo Clinic, Rochester, Minnesota 55905, USA. 5 Mayo Translational PKD Center, Mayo Clinic, Rochester, Minnesota 55905, USA. -

Unraveling the Genetics of Joubert and Meckel-Gruber Syndromes
Journal of Pediatric Genetics 3 (2014) 65–78 65 DOI 10.3233/PGE-14090 IOS Press Unraveling the genetics of Joubert and Meckel-Gruber syndromes Katarzyna Szymanska, Verity L. Hartill and Colin A. Johnson∗ Department of Ophthalmology and Neuroscience, University of Leeds, Leeds, UK Received 27 May 2014 Revised 11 July 2014 Accepted 14 July 2014 Abstract. Joubert syndrome (JBTS) and Meckel-Gruber syndrome (MKS) are recessive neurodevelopmental conditions caused by mutations in proteins that are structural or functional components of the primary cilium. In this review, we provide an overview of their clinical diagnosis, management and molecular genetics. Both have variable phenotypes, extreme genetic heterogeneity, and display allelism both with each other and other ciliopathies. Recent advances in genetic technology have significantly improved diagnosis and clinical management of ciliopathy patients, with the delineation of some general genotype-phenotype correlations. We highlight those that are most relevant for clinical practice, including the correlation between TMEM67 mutations and the JBTS variant phenotype of COACH syndrome. The subcellular localization of the known MKS and JBTS proteins is now well-described, and we discuss some of the contemporary ideas about ciliopathy disease pathogenesis. Most JBTS and MKS proteins localize to a discrete ciliary compartment called the transition zone, and act as structural components of the so-called “ciliary gate” to regulate the ciliary trafficking of cargo proteins or lipids. Cargo proteins include enzymes and transmembrane proteins that mediate intracellular signaling. The disruption of transition zone function may contribute to the ciliopathy phenotype by altering the composition of the ciliary membrane or axoneme, with impacts on essential developmental signaling including the Wnt and Shh pathways as well as the regulation of secondary messengers such as inositol-1,4,5-trisphosphate (InsP3) and cyclic adenosine monophosphate (cAMP). -

Ciliopathies Gene Panel
Ciliopathies Gene Panel Contact details Introduction Regional Genetics Service The ciliopathies are a heterogeneous group of conditions with considerable phenotypic overlap. Levels 4-6, Barclay House These inherited diseases are caused by defects in cilia; hair-like projections present on most 37 Queen Square cells, with roles in key human developmental processes via their motility and signalling functions. Ciliopathies are often lethal and multiple organ systems are affected. Ciliopathies are London, WC1N 3BH united in being genetically heterogeneous conditions and the different subtypes can share T +44 (0) 20 7762 6888 many clinical features, predominantly cystic kidney disease, but also retinal, respiratory, F +44 (0) 20 7813 8578 skeletal, hepatic and neurological defects in addition to metabolic defects, laterality defects and polydactyly. Their clinical variability can make ciliopathies hard to recognise, reflecting the ubiquity of cilia. Gene panels currently offer the best solution to tackling analysis of genetically Samples required heterogeneous conditions such as the ciliopathies. Ciliopathies affect approximately 1:2,000 5ml venous blood in plastic EDTA births. bottles (>1ml from neonates) Ciliopathies are generally inherited in an autosomal recessive manner, with some autosomal Prenatal testing must be arranged dominant and X-linked exceptions. in advance, through a Clinical Genetics department if possible. Referrals Amniotic fluid or CV samples Patients presenting with a ciliopathy; due to the phenotypic variability this could be a diverse set should be sent to Cytogenetics for of features. For guidance contact the laboratory or Dr Hannah Mitchison dissecting and culturing, with ([email protected]) / Prof Phil Beales ([email protected]) instructions to forward the sample to the Regional Molecular Genetics Referrals will be accepted from clinical geneticists and consultants in nephrology, metabolic, laboratory for analysis respiratory and retinal diseases. -

American Board of Psychiatry and Neurology, Inc
AMERICAN BOARD OF PSYCHIATRY AND NEUROLOGY, INC. CERTIFICATION EXAMINATION IN NEUROLOGY 2015 Content Blueprint (January 13, 2015) Part A Basic neuroscience Number of questions: 120 01. Neuroanatomy 3-5% 02. Neuropathology 3-5% 03. Neurochemistry 2-4% 04. Neurophysiology 5-7% 05. Neuroimmunology/neuroinfectious disease 2-4% 06. Neurogenetics/molecular neurology, neuroepidemiology 2-4% 07. Neuroendocrinology 1-2% 08. Neuropharmacology 4-6% Part B Behavioral neurology, cognition, and psychiatry Number of questions: 80 01. Development through the life cycle 3-5% 02. Psychiatric and psychological principles 1-3% 03. Diagnostic procedures 1-3% 04. Clinical and therapeutic aspects of psychiatric disorders 5-7% 05. Clinical and therapeutic aspects of behavioral neurology 5-7% Part C Clinical neurology (adult and child) The clinical neurology section of the Neurology Certification Examination is comprised of 60% adult neurology questions and 40% child neurology questions. Number of questions: 200 01. Headache disorders 1-3% 02. Pain disorders 1-3% 03. Epilepsy and episodic disorders 1-3% 04. Sleep disorders 1-3% 05. Genetic disorders 1-3% 2015 ABPN Content Specifications Page 1 of 22 Posted: Certification in Neurology AMERICAN BOARD OF PSYCHIATRY AND NEUROLOGY, INC. 06. Congenital disorders 1-3% 07. Cerebrovascular disease 1-3% 08. Neuromuscular diseases 2-4% 09. Cranial nerve palsies 1-3% 10. Spinal cord diseases 1-3% 11. Movement disorders 1-3% 12. Demyelinating diseases 1-3% 13. Neuroinfectious diseases 1-3% 14. Critical care 1-3% 15. Trauma 1-3% 16. Neuro-ophthalmology 1-3% 17. Neuro-otology 1-3% 18. Neurologic complications of systemic diseases 2-4% 19. -

Joubert Syndrome and Related Disorders
Brancati et al. Orphanet Journal of Rare Diseases 2010, 5:20 http://www.ojrd.com/content/5/1/20 REVIEW Open Access JoubertReview Syndrome and related disorders Francesco Brancati1,2, Bruno Dallapiccola3 and Enza Maria Valente*1,4 Abstract Joubert syndrome (JS) and related disorders (JSRD) are a group of developmental delay/multiple congenital anomalies syndromes in which the obligatory hallmark is the molar tooth sign (MTS), a complex midbrain-hindbrain malformation visible on brain imaging, first recognized in JS. Estimates of the incidence of JSRD range between 1/80,000 and 1/ 100,000 live births, although these figures may represent an underestimate. The neurological features of JSRD include hypotonia, ataxia, developmental delay, intellectual disability, abnormal eye movements, and neonatal breathing dysregulation. These may be associated with multiorgan involvement, mainly retinal dystrophy, nephronophthisis, hepatic fibrosis and polydactyly, with both inter- and intra-familial variability. JSRD are classified in six phenotypic subgroups: Pure JS; JS with ocular defect; JS with renal defect; JS with oculorenal defects; JS with hepatic defect; JS with orofaciodigital defects. With the exception of rare X-linked recessive cases, JSRD follow autosomal recessive inheritance and are genetically heterogeneous. Ten causative genes have been identified to date, all encoding for proteins of the primary cilium or the centrosome, making JSRD part of an expanding group of diseases called "ciliopathies". Mutational analysis of causative genes is available in few laboratories worldwide on a diagnostic or research basis. Differential diagnosis must consider in particular the other ciliopathies (such as nephronophthisis and Senior-Loken syndrome), distinct cerebellar and brainstem congenital defects and disorders with cerebro-oculo-renal manifestations. -

Renal Cystic Disorders Infosheet 6-14-19
Next Generation Sequencing Panel for Renal Cystic Disorders Clinical Features: Renal cystic diseases are a genetically heterogeneous group of conditions characterized By isolated renal disease or renal cysts in conjunction with extrarenal features (1). Age of onset of renal cystic disease ranges from neonatal to adult onset. Common features of renal cystic diseases include renal insufficiency and progression to end stage renal disease (ESRD). Identification of the genetic etiology of renal cystic disease can aid in appropriate clinical management of the affected patient. Our Renal Cystic Disorders Panel includes sequence and deletion/duplicaton analysis of all 79 genes listed below. Renal Cystic Disorders Sequencing Panel AHI1 BMPER HNF1B NEK8 TCTN3 WDPCP ANKS6 C5orf42 IFT27 NOTCH2 TFAP2A WDR19 ARL13B CC2D2A IFT140 NPHP1 TMEM107 XPNPEP3 ARL6 CDC73 IFT172 NPHP3 TMEM138 ZNF423 B9D1 CEP104 INPP5E NPHP4 TMEM216 B9D2 CEP120 INVS OFD1 TMEM231 BBIP1 CEP164 IQCB1 PDE6D TMEM237 BBS1 CEP290 JAG1 PKD2 TMEM67 BBS10 CEP41 KIAA0556 PKHD1 TRIM32 BBS12 CEP83 KIAA0586 REN TSC1 BBS2 CRB2 KIF14 RPGRIP1L TSC2 BBS4 CSPP1 KIF7 SALL1 TTC21B BBS5 DCDC2 LZTFL1 SDCCAG8 TTC8 BBS7 GLIS2 MKKS TCTN1 UMOD BBS9 GLIS3 MKS1 TCTN2 VHL Disorder Genes Inheritance Clinical features/molecular genetics Bardet Biedl ARL6 AR Bardet-Biedl syndrome (BBS) is an autosomal syndrome BBS1 recessive multi-systemic ciliopathy characterized By BBS10 retinal dystrophy, oBesity, postaxial polydactyly, BBS12 leaning difficulties, renal involvement and BBS2 genitourinary abnormalities (2). Visual prognosis is BBS4 poor, and the mean age of legal Blindness is 15.5 BBS5 years. Birth weight is typically normal But significant BBS7 weight gain Begins within the first year. Renal BBS9 disease is a major cause of morBidity and mortality. -

Autism Spectrum Disorders—A Genetics Review Judith H
GENETEST REVIEW Genetics in Medicine Autism spectrum disorders—A genetics review Judith H. Miles, MD, PhD TABLE OF CONTENTS Prevalence .........................................................................................................279 Adenylosuccinate lyase deficiency ............................................................285 Clinical features................................................................................................279 Creatine deficiency syndromes..................................................................285 Core autism symptoms...................................................................................279 Smith-Lemli-Opitz syndrome.....................................................................285 Diagnostic criteria and tools..........................................................................280 Other single-gene disorders.......................................................................285 Neurologic and medical symptoms .............................................................281 Developmental syndromes of undetermined etiology..............................286 Genetics of autism...........................................................................................281 Moebius syndrome or sequence...............................................................286 Chromosomal disorders and CNVS..............................................................282 Landau-Kleffner syndrome .........................................................................286 Single-gene -

Pallister–Hall Syndrome
1-10-2020 Pallister–Hall Syndrome J Pediatr Neurosci. 2017 Jul-Sep; 12(3): 276–279. PMCID: PMC5696670 doi: 10.4103/jpn.JPN_101_17: 10.4103/jpn.JPN_101_17 PMID: 29204208 Pallister–Hall Syndrome Sadanandvalli Retnaswami Chandra, Mane Maheshkumar Daryappa,1 M. A. Mukheem Mudabbir, 1 M. Pooja, and A. Arivazhagan Neurocentre, National Institute of Mental Health and Neurosciences, Bengaluru, Karnataka, India 1Department of Neurology, National Institute of Mental Health and Neurosciences, Bengaluru, Karnataka, India Address for correspondence: Dr. Sadanandavalli Retnaswami Chandra, Department of Neurology, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India. E-mail: [email protected] Copyright : © 2017 Journal of Pediatric Neurosciences This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial- ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms. Abstract Polydactyly is a relatively common abnormality in infants. However, it can be a marker of a wide variety of neurological and systemic abnormality. Hence, it is important for pediatrician and physician to have insight into the various association of this apparently innocuous anomaly. In this write-up, we report an extremely rare syndrome associated with polydactyly that is Pallister–Hall syndrome. A 10-month-old male child born by lower segment cesarean section presented with global delay associated with microcephaly, frontal bossing, hypertelorism, flat nose, short philtrum, incomplete cleft in the upper lip and hard palate, polydactyly, and syndactyly. The child presented with repeated vomiting and crying episodes. -
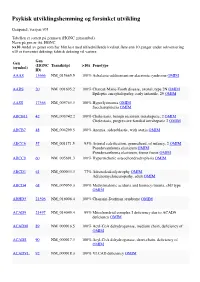
Psykisk Utviklingshemming Og Forsinket Utvikling
Psykisk utviklingshemming og forsinket utvikling Genpanel, versjon v03 Tabellen er sortert på gennavn (HGNC gensymbol) Navn på gen er iht. HGNC >x10 Andel av genet som har blitt lest med tilfredstillende kvalitet flere enn 10 ganger under sekvensering x10 er forventet dekning; faktisk dekning vil variere. Gen Gen (HGNC Transkript >10x Fenotype (symbol) ID) AAAS 13666 NM_015665.5 100% Achalasia-addisonianism-alacrimia syndrome OMIM AARS 20 NM_001605.2 100% Charcot-Marie-Tooth disease, axonal, type 2N OMIM Epileptic encephalopathy, early infantile, 29 OMIM AASS 17366 NM_005763.3 100% Hyperlysinemia OMIM Saccharopinuria OMIM ABCB11 42 NM_003742.2 100% Cholestasis, benign recurrent intrahepatic, 2 OMIM Cholestasis, progressive familial intrahepatic 2 OMIM ABCB7 48 NM_004299.5 100% Anemia, sideroblastic, with ataxia OMIM ABCC6 57 NM_001171.5 93% Arterial calcification, generalized, of infancy, 2 OMIM Pseudoxanthoma elasticum OMIM Pseudoxanthoma elasticum, forme fruste OMIM ABCC9 60 NM_005691.3 100% Hypertrichotic osteochondrodysplasia OMIM ABCD1 61 NM_000033.3 77% Adrenoleukodystrophy OMIM Adrenomyeloneuropathy, adult OMIM ABCD4 68 NM_005050.3 100% Methylmalonic aciduria and homocystinuria, cblJ type OMIM ABHD5 21396 NM_016006.4 100% Chanarin-Dorfman syndrome OMIM ACAD9 21497 NM_014049.4 99% Mitochondrial complex I deficiency due to ACAD9 deficiency OMIM ACADM 89 NM_000016.5 100% Acyl-CoA dehydrogenase, medium chain, deficiency of OMIM ACADS 90 NM_000017.3 100% Acyl-CoA dehydrogenase, short-chain, deficiency of OMIM ACADVL 92 NM_000018.3 100% VLCAD -

Blueprint Genetics Comprehensive Growth Disorders / Skeletal
Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders Panel Test code: MA4301 Is a 374 gene panel that includes assessment of non-coding variants. This panel covers the majority of the genes listed in the Nosology 2015 (PMID: 26394607) and all genes in our Malformation category that cause growth retardation, short stature or skeletal dysplasia and is therefore a powerful diagnostic tool. It is ideal for patients suspected to have a syndromic or an isolated growth disorder or a skeletal dysplasia. About Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders This panel covers a broad spectrum of diseases associated with growth retardation, short stature or skeletal dysplasia. Many of these conditions have overlapping features which can make clinical diagnosis a challenge. Genetic diagnostics is therefore the most efficient way to subtype the diseases and enable individualized treatment and management decisions. Moreover, detection of causative mutations establishes the mode of inheritance in the family which is essential for informed genetic counseling. For additional information regarding the conditions tested on this panel, please refer to the National Organization for Rare Disorders and / or GeneReviews. Availability 4 weeks Gene Set Description Genes in the Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders Panel and their clinical significance Gene Associated phenotypes Inheritance ClinVar HGMD ACAN# Spondyloepimetaphyseal dysplasia, aggrecan type, AD/AR 20 56 Spondyloepiphyseal dysplasia, Kimberley -

Clinical Utility Gene Card For: Joubert Syndrome - Update 2013
European Journal of Human Genetics (2013) 21, doi:10.1038/ejhg.2013.10 & 2013 Macmillan Publishers Limited All rights reserved 1018-4813/13 www.nature.com/ejhg CLINICAL UTILITY GENE CARD UPDATE Clinical utility gene card for: Joubert syndrome - update 2013 Enza Maria Valente*,1,2, Francesco Brancati1, Eugen Boltshauser3 and Bruno Dallapiccola4 European Journal of Human Genetics (2013) 21, doi:10.1038/ejhg.2013.10; published online 13 February 2013 Update to: European Journal of Human Genetics (2011) 19, doi:10.1038/ejhg.2011.49; published online 30 March 2011 1. DISEASE CHARACTERISTICS 1.6 Analytical methods 1.1 Name of the disease (synonyms) Direct sequencing of coding genomic regions and splice site junctions; Joubert syndrome (JS); Joubert-Boltshauser syndrome; Joubert syn- multiplex microsatellite analysis for detection of NPHP1 homozygous drome-related disorders (JSRD), including cerebellar vermis hypo/ deletion. Possibly, qPCR or targeted array-CGH for detection of aplasia, oligophrenia, congenital ataxia, ocular coloboma, and hepatic genomic rearrangements in other genes. fibrosis (COACH) syndrome; cerebellooculorenal, or cerebello-oculo- renal (COR) syndrome; Dekaban-Arima syndrome; Va´radi-Papp 1.7 Analytical validation syndrome or Orofaciodigital type VI (OFDVI) syndrome; Malta Direct sequencing of both DNA strands; verification of sequence and syndrome. qPCR results in an independent experiment. 1.2 OMIM# of the disease 1.8 Estimated frequency of the disease 213300, 243910, 216360, 277170. (incidence at birth-‘birth prevalence’-or population prevalence) No good population-based data on JSRD prevalence have been published. A likely underestimated frequency between 1/80 000 and 1.3 Name of the analysed genes or DNA/chromosome segments 1/100 000 live births is based on unpublished data.