Biofilm-Based Nosocomial Infections
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ISPA International Society for the Performing Arts
International Society for the ISPA Performing Arts TODAY’S FUTURE FOR THE ARTS NEW YORK CONGRESS JANUARY 8–10, 2019 Theatre buildings by theatre people for theatre people. Clockwise from top left: Linbury Theatre, Royal Opera House; The Yard at Chicago Shakespeare Theater; Hudson Theatre, Broadway; Studio Theatre, Bristol Old Vic. MESSAGE FROM THE CHAIR OF ISPA Dear Friends, ransitions. We all think about them. Some of us worry about them. But we are all impacted by them. As much as we may try to be present each moment of our days, it is the rare person who does not ponder what comes next. Transitions can be as exhilarating as they are scary. In these times of rapid-fire change and volatility (mostly beyond our personal control), it is Teasy to default to a position of wanting to hang on to what we know. Stay the course. Be steady. Reduce risk. WELCOME Yet continuing to do the same thing that works for us today may be the very act that fails us tomorrow. Our Congress Co-chairs Collette Brennan and Steinunn Ragnarsdóttir have given us a brilliantly provocative theme to consider with an array of thought-leaders from within our field and beyond offering varied perspectives on how we might navigate the inevitability of the transitions we encounter. For me, transitions is a particularly apt theme as I prepare to pass on the chairmanship of ISPA to our colleague Tisa Ho. As an ISPA member for 20 years and board member for most of that time, I have been fortunate to work with so many gifted members who have given selflessly in leading ISPA through incredible Transitions. -

The Action of Selected Isothiocyanates on Bacterial Biofilm Prevention and Control
This article was published in International Biodeterioration & Biodegradation 86, 25-33, 2014 http://dx.doi.org/10.1016/j.ibiod.2013.01.015 The action of selected isothiocyanates on bacterial biofilm prevention and control Anabela Borges a,b, Lúcia C. Simões a,c, Maria J. Saavedra b, Manuel Simões a,* a LEPAE, Department of Chemical Engineering, Faculty of Engineering, University of Porto, Rua Dr. Roberto Frias, s/n, 4200-465 Porto, Portugal b CECAV e Veterinary and Animal Science Research Center, Veterinary Science Department, University of Trás-os-Montes e Alto Douro, Apartado 1013, 5001- 801 Vila Real, Portugal c IBB e Institute for Biotechnology and Bioengineering, Centre of Biological Engineering, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal Abstract The activity of two selected isothiocyanates (ITCs), allylisothiocyanate (AITC) and 2-phenylethy- lisothiocyanate (PEITC) was evaluated on the prevention and control of biofilms formed by Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Listeria monocytogenes. In addition, the effect of ITCs was also tested on planktonic cell susceptibility, bacterial motility and adhesion. Biofilm prevention and control were tested using a microtiter plate assay and the effect of ITCs was assessed on biofilm mass and metabolic activity. The minimum bactericidal concentration for E. coli and P. aeruginosa was 1000 g mL-1 (AITC) and > 1000 g mL-1 (PEITC), for S. aureus and L. monocytogenes was > 1000 g mL-1 (for both ITCs). AITC caused total inhibition of swimming (P. aeruginosa) and swarming (E. coli) motilities. PEITC caused total inhibition of swimming (E. coli, P. aeruginosa and L. monocytogenes) and swarming (E. -

Public Poll Findings and Methodology
PUBLIC POLL FINDINGS AND METHODOLOGY Misinformation around U.S. Capitol unrest, election spreading among Americans New Ipsos poll shows Capitol rioters viewed as “criminals,” “right-wing terrorists,” but views divided sharply along partisan lines Topline Findings Washington, DC, January 19, 2021 – In the wake of the January 6th storming of the U.S. Capitol, Ipsos took another look at beliefs toward misinformation and election-related conspiracy theories. There is over a 60-point gap between Democrats and Republicans regarding whether Joe Biden legitimately won the 2020 presidential election, a sign of how politically divided this country is, and what facts we are willing to accept. Following the attack last week, most Americans see the people who entered the Capitol as criminals or right-wing domestic terrorists and, as a result, support the suspension of the president’s social media accounts. Detailed Findings 1. Most of the American public views the people who entered the Capitol as criminals or right-wing domestic terrorists. A plurality support social media outlets removing President Trump and QAnon accounts as a result of the event. • Nearly one in three (31%) say they are criminals, and 26% say they are right-wing terrorists. • Just four percent believe they are patriots who were fighting to preserve our freedoms. • News consumption plays a role in views of the events, as does partisanship. • For example, one in four who get their news from FOX or conservative online outlets believe they were left-wing terrorists or Antifa, compared to just 7% of CNN or MSNBC viewers. Forty-five percent of Democrats see these people as right-wing domestic terrorists, while 10% of Republicans feel the same. -

Partisan Platforms: Responses to Perceived Liberal Bias in Social Media
Partisan Platforms: Responses to Perceived Liberal Bias in Social Media A Research Paper submitted to the Department of Engineering and Society Presented to the Faculty of the School of Engineering and Applied Science University of Virginia • Charlottesville, Virginia In Partial Fulfillment of the Requirements for the Degree Bachelor of Science, School of Engineering Luke Giraudeau Spring, 2021 On my honor as a University Student, I have neither given nor received unauthorized aid on this assignment as defined by the Honor Guidelines for Thesis-Related Assignments Signature __________________________________________ Date __________ Luke Giraudeau Approved __________________________________________ Date __________ Richard Jacques, Department of Engineering and Society Introduction In the United States, public opinion about tech companies’ political biases is divided along partisan lines (Vogels, Perrin, & Anderson, 2020). In the U.S. since 2018, 69 percent of Republicans claim that technology companies favor liberal views, whereas only 19 percent of Democrats say that technology companies favor the alternative view. Over 50 percent of liberals believe that perspectives are treated equally, whereas only 22 percent of conservatives feel this way. Critics who allege bias have organized to promote legislation such as the Ending Support for Internet Censorship Act (2020) as well as an executive order (Executive Order 13,925, 2020). Furthermore, conservative entrepreneurs have produced new social media platforms such as Gab and Parler that claim -

Mewe® Launches Journals — the Future of Photo/Video Albums Rapidly Growing Facebook Competitor Gives Members a Fun
® MeWe Launches Journals — The Future of Photo/Video Albums Rapidly growing Facebook competitor gives members a fun and unique way to organize, save, and share their Stories LOS ANGELES, June 29, 2020 – Tired of seeing your favorite SNAP and INSTA Stories ® disappear? Join the MeWe movement! Upstart MeWe , the rapidly growing “Anti-Facebook” with data privacy, free speech and no ads, today launches Journals – the first feature of its kind on any social network. The idea for Journals was born in the ascent of Stories. In recent years, Stories have become social media's favored 24-hour lifecycle medium for creating and sharing brief videos of special moments and daily life events. With MeWe Journals, members can save, sort, and organize their favorite Stories beyond the moment - for a lifetime of memories and sharing. MeWe Journals Allow Members To: ● Organize and aggregate their favorite Stories into Journals on their profiles ● Sort Stories by friend or family member, topic, timeline, or anyway they’d like ● Choose to make each Journal visible to only themselves; their contacts; Close Friends; or open to anyone on MeWe ● Create up to 500 Journals, and each Journal can contain up to 1,000 Stories MeWe Journals can be created and edited on MeWe’s mobile app (available on iOS and Android), and then can be viewed on both mobile and desktop. Journals are included with MeWe Premium ($4.99 per month) or can be purchased a-la-carte in the MeWe Store for $1.99 per month. MeWe members given view access by a Journal creator can see that member’s Journal without subscribing. -

New Titles Spring 2021 Spring Titles New
SUBJECTS NOG AANPASSEN JOHN BENJAMINS PUBLISHING COMPANY P.O. Box 36224 nl-1020 me Amsterdam / The Netherlands SPRING 2021 Linguistics Literary studies Philosophy Translation studies John Benjamins Publishing Company catalog.NT.2021.SPRING.cover.indd 1 11/02/2021 12:25:55 JOHN BENJAMINS PUBLISHING COMPANY AMSTERDAM/PHILADELPHIA www.benjamins.com Main office (all customers except North America) Customers in North America John Benjamins Publishing Company Orders & Payments Klaprozenweg 75g John Benjamins Publishing Company P.O. Box 36224 P.O. Box 960 nl-1020 me Amsterdam Herndon VA 20172-0960, USA The Netherlands Tel.: 800 562-5666 0 Fax 703 661-1501 Tel. +31 20 6304747 / Fax +31 20 6739773 For book orders: [email protected] For book/e-book orders: [email protected] Returns Department John Benjamins Publishing Company For E-mail queries: 22880 Quicksilver Drive [email protected] Dulles VA 20166, USA General Ordering Information • Postage and packing will be charged additionally. • All books may be ordered through your regular bookseller or For a summary of these charges: directly from the publisher. Orders from customers in the U.S.A., www.benjamins.com/content/customers/bookorders Canada and Mexico will be filled by our office in Herndon VA. • For sales within the European Union, VAT will be charged (U.S.A.). Customers from countries where we have exclusive unless a VAT number is supplied with the order. distributors should order from these directly. Customers from all other territories should direct their orders to our office in • For sales within the U.S.A. local sales tax, if applicable, will Amsterdam, The Netherlands. -

Netclean Report 2019
NETCLEAN REPORT 2019 A REPORT ABOUT CHILD SEXUAL ABUSE CRIME 1 INTRODUCTION INTRODUCTION p. 4–5 EXECUTIVE SUMMARY p. 6–7 ABOUT THE REPORT p. 8–9 RESULTS EIGHT INSIGHTS INTO CHILD SEXUAL ABUSE CRIME p. 10–11 PART ONE: LAW ENFORCEMENT SURVEY p. 12–13 1. The spread of live-streamed child sexual abuse p. 14–17 2. Victims of live-streamed child sexual abuse p. 18–19 3. Offenders who consume live-streamed child sexual abuse p. 20–25 4. How child sexual abuse material is stored p. 28–31 5. Apps and platforms are used to store and distribute child sexual abuse material p. 32–33 6. Emerging technologies – trends, challenges and opportunities p. 36–40 PART TWO: BUSINESS SURVEY p. 44–45 7. Businesses’ use of policies and action plans to protect their IT environment from child sexual abuse material p. 46–47 8. Businesses’ use of technologies to protect their IT environment from child sexual abuse material p. 48–49 PART THREE: MAPPING OF TECHNOLOGIES p. 52–53 Binary hashing p. 54 Robust hashing p. 55 Artificial Intelligence p. 56 Keyword matching p. 57 Filter technology p. 58 Blocking technology p. 59 IN CLOSING TECHNOLOGY – A DRIVER OF BOTH PROBLEM AND SOLUTION p. 60 SAFEGUARDED CHILDREN IN 2018 AND ACKNOWLEDGEMENTS p. 62 2 3 INTRODUCTION BY USING TECHNOLOGY TO About John F. Clark and NCMEC John F. Clark is president and CEO of the National Center OUR ADVANTAGE WE for Missing & Exploited Children (NCMEC). Clark has an extensive law-enforcement background, including 28 years with the United States Marshals Service (USMS). -

ADVISORY BOARD March 2020
1 ADVISORY BOARD March 2020 Sir Tim Berners-Lee Inventor of the World Wide Web Tim Berners-Lee invented the World Wide Web in 1989. He wrote the first web client and server in 1990. His specifications of URIs, HTTP and HTML were refined as Web technology spread. He is the Director of the World Wide Web Consortium (W3C), a Web standards organization founded in 1994 that develops interoperable technologies (specifications, guidelines, software, and tools) to lead the Web to its full potential. He is the 3Com Founders Professor of Engineering in the School of Engineering with a joint appointment in the Department of Electrical Engineering and Computer Science at the Laboratory for Computer Science and Artificial Intelligence (CSAIL) at the Massachusetts Institute of Technology (MIT) where he also heads the Decentralized Information Group (DIG). Tim is a Director of the World Wide Web Foundation and is a member of the UK's Transparency Board, and president of London's Open Data Institute. Dany Garcia CEO, The Garcia Companies and Seven Bucks Productions As Chairwoman and CEO of The Garcia Companies, Dany is the visionary architect behind some of today’s most successful enterprises, brands and talent. In this capacity, she is the very successful manager of Dwayne “The Rock” Johnson. In addition, Dany is the CEO of Seven Bucks Productions, a full-service production company she and Johnson co-founded. Dany is the recipient of many awards and recognitions, and her passion for bettering the world through socially responsible decisions is consistently reflected in the culture of her teams and the businesses she builds. -

Building Resilience & Confronting Risk
BUILDING RESILIENCE & CONFRONTING RISK A PARENTS & CAREGIVERS GUIDE TO ONLINE RADICALIZATION POLARIZATION AND EXTREMISM RESEARCH AND INNOVATION LAB (PERIL) PERIL brings the resources and expertise of the university sector to bear on the problem of growing youth polarization and extremist radicalization, through scalable research, intervention, and public education ideas to reduce rising polarization and hate. SOUTHERN POVERTY LAW CENTER The SPLC seeks to be a catalyst for racial justice in the South and beyond, working in partnership with communities to dismantle white supremacy, strengthen intersectional movements, and advance the human rights of all people. CONTENTS PARENTS & CAREGIVERS GUIDE 3 WHAT IS ONLINE RADICALIZATION? WHY SHOULD YOU CARE? 4 RECOGNIZING WARNING SIGNS 5 UNDERSTANDING THE DRIVERS 6 ENGAGE & EMPOWER 8 RESPONDING TO HATE 10 HOW TO GET HELP 11 APPENDIX: STAYING ALERT TO SITES, PLATFORMS, & APPS FREQUENTLY EXPLOITED BY EXTREMISTS 15 ENDNOTES 16 CREDITS 17 ILLUSTRATIONS BY CLAUDIA WHITAKER Whether you live with a young person, or now work virtually with youth, radicalization to extremism is something we all should be concerned about. Extremists looking to recruit and convert children are predatory. Like all forms of child exploitation, extremist recruitment drives a wedge between young people and the adults they would typically trust. Radicalization is a problem for our entire society, from the innocent people it victimizes to the family bonds it breaks apart. 2 A PARENTS AND CAREGIVERS GUIDE TO ONLINE RADICALIZATION PARENTS & CAREGIVERS GUIDE Who is this guide for? We wrote this guide with a wide range Whether you live with a young person or work with youth of caregivers in mind. -
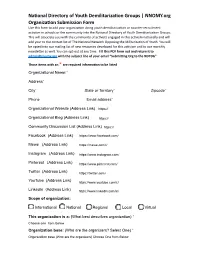
National Directory of Youth Demilitarization Groups | NNOMY.Org
National Directory of Youth Demilitarization Groups | NNOMY.org Organization Submission Form Use this form to add your organization doing youth demilitarization or counter-recruitment activism in schools or the community into the National Directory of Youth Demilitarization Groups. This will associate you with the community of activists engaged in this activism nationally and will add your to the contact list of The National Network Opposing the Militarization of Youth. You will be opted into our mailing list of new resources developed for this activism and to our monthly newsletter as well. You can opt-out at any time. Fill this PDF form out and return it to [email protected] with the subject line of your email “Submitting Org to the NDYDG” Those items with an * are required information to be listed Organizational Name:* Address* City* State or Territory* Zipcode* Phone Email address* Organizational Website (Address Link) Organizational Blog (Address Link) Community Discussion List (Address Link) Facebook (Address Link) Mewe (Address Link) Instagram (Address Link) Pinterest (Address Link) Twitter (Address Link) YouTube (Address Link) LinkedIn (Address Link) Scope of organization: International National Regional Local Virtual This organization is a: (What best describes organization) * Organization base: (Who are the organizers? Select One) * National Directory of Youth Demilitarization Groups | NNOMY.org Organization Submission Form Outreach to specific communities: (You can select multiples here) African American Asian Arab Educators Faith -

Mechanism of Candida Albicans Biofilm and Virulence Inhibition by a Bacterial Secreted Factor
The Texas Medical Center Library DigitalCommons@TMC The University of Texas MD Anderson Cancer Center UTHealth Graduate School of The University of Texas MD Anderson Cancer Biomedical Sciences Dissertations and Theses Center UTHealth Graduate School of (Open Access) Biomedical Sciences 12-2017 MECHANISM OF CANDIDA ALBICANS BIOFILM AND VIRULENCE INHIBITION BY A BACTERIAL SECRETED FACTOR Carrie Graham Follow this and additional works at: https://digitalcommons.library.tmc.edu/utgsbs_dissertations Part of the Immunology and Infectious Disease Commons, Medicine and Health Sciences Commons, and the Pathogenic Microbiology Commons Recommended Citation Graham, Carrie, "MECHANISM OF CANDIDA ALBICANS BIOFILM AND VIRULENCE INHIBITION BY A BACTERIAL SECRETED FACTOR" (2017). The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access). 826. https://digitalcommons.library.tmc.edu/utgsbs_dissertations/826 This Dissertation (PhD) is brought to you for free and open access by the The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences at DigitalCommons@TMC. It has been accepted for inclusion in The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access) by an authorized administrator of DigitalCommons@TMC. For more information, please contact [email protected]. MECHANISM OF CANDIDA ALBICANS BIOFILM AND VIRULENCE INHIBITION BY A BACTERIAL SECRETED -

Mark Weinstein Is the Founder and Chief Evangelist of Mewe. Mark Is World Renowned As a Leading Privacy Advocate and One Of
Mark Weinstein is the Founder and Chief Evangelist of MeWe. Mark is world renowned as a leading privacy advocate and one of the visionary inventors of social networking. Mark’s TED Talk in March 2020: “The Rise of Surveillance Capitalism”. Mark is ranked one of the "Top 8 Minds in Online Privacy," and was named “Privacy by Design Ambassador” by The Information and Privacy Commissioner of Ontario, Canada. Mark's articles about privacy and social media have appeared in The Wall Street Journal, NY Post, The Mirror (UK), HuffPost, USA Today, and many others. He has been interviewed on CNN, Fox News, Fox Business, BBC, PBS, etc. Mark has been a featured speaker and social media/privacy expert on the stage at many conferences around the globe, including EY’s Strategic Growth Forum, KNOW Identity Conference, Global Security Conference, GMIC New York, Security BSides Vancouver, and Customer Experience Asia. Back in 1998, Mark created SuperFamily.com and SuperFriends.com, which were ranked by PC Magazine as “Top 100” sites 3 years in a row. Mark sold those companies in the early 2000s and then built an executive coaching company, also giving keynotes and authoring books on personal and professional greatness, titled “Habitually Great", which won 2 Indie Book awards and were endorsed by Stephen Covey. A decade later, as Facebook began to dominate social media, Mark Zuckerberg stated, “Privacy is a social norm of the past,” and that statement made Mark Weinstein's jaw drop. With guidance from MeWe Advisor, Sir Tim Berners-Lee (the inventor of the Web), Mark endeavored to build the next-generation social media experience that gives people all the great features of social media while eliminating spying, targeting, newsfeed manipulation, and undermining democracy.