Human and Porcine Hepatitis E Virus Strains, United Kingdom
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
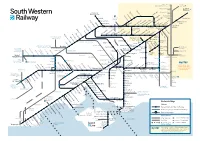
Download Network
Milton Keynes, London Birmingham and the North Victoria Watford Junction London Brentford Waterloo Syon Lane Windsor & Shepherd’s Bush Eton Riverside Isleworth Hounslow Kew Bridge Kensington (Olympia) Datchet Heathrow Chiswick Vauxhall Airport Virginia Water Sunnymeads Egham Barnes Bridge Queenstown Wraysbury Road Longcross Sunningdale Whitton TwickenhamSt. MargaretsRichmondNorth Sheen BarnesPutneyWandsworthTown Clapham Junction Staines Ashford Feltham Mortlake Wimbledon Martins Heron Strawberry Earlsfield Ascot Hill Croydon Tramlink Raynes Park Bracknell Winnersh Triangle Wokingham SheppertonUpper HallifordSunbury Kempton HamptonPark Fulwell Teddington Hampton KingstonWick Norbiton New Oxford, Birmingham Winnersh and the North Hampton Court Malden Thames Ditton Berrylands Chertsey Surbiton Malden Motspur Reading to Gatwick Airport Chessington Earley Bagshot Esher TolworthManor Park Hersham Crowthorne Addlestone Walton-on- Bath, Bristol, South Wales Reading Thames North and the West Country Camberley Hinchley Worcester Beckenham Oldfield Park Wood Park Junction South Wales, Keynsham Trowbridge Byfleet & Bradford- Westbury Brookwood Birmingham Bath Spaon-Avon Newbury Sandhurst New Haw Weybridge Stoneleigh and the North Reading West Frimley Elmers End Claygate Farnborough Chessington Ewell West Byfleet South New Bristol Mortimer Blackwater West Woking West East Addington Temple Meads Bramley (Main) Oxshott Croydon Croydon Frome Epsom Taunton, Farnborough North Exeter and the Warminster Worplesdon West Country Bristol Airport Bruton Templecombe -

White Cottage Ottershaw • Surrey White Cottage Murray Road, Ottershaw, Surrey, Kt16 0Hp
WHITE COTTAGE OTTERSHAW • SURREY WHITE COTTAGE MURRAY ROAD, OTTERSHAW, SURREY, KT16 0HP A stunning contemporary family home, thoughtfully designed for entertaining as well as modern family living. Beautiful views Perfect for entertaining Light and bright accommodation Lateral living Gated driveway DESCRIPTION A fabulous modern family home, built in 2011 by the current owners. The modern design of this house allows light to flood in and provides excellent balanced accommodation for comfortable family living as well as outstanding entertaining space. Approached via electric gates, the property has been thoughtfully designed and features underfloor heating throughout, air ventilation and a control 4 system. The front door leads into the striking entrance hall. To the right is the true hub of this home, the open plan kitchen/breakfast/ dining/living room with corner bi-fold doors that open up to give breath-taking views of the gardens and fields beyond. Clever use of decking joins the indoor living space with the outdoors. The master bedroom suite is unique with its open plan en suite shower room overlooking the rear garden and views beyond and a walk in wardrobe. 2 further bedrooms, both with access out to the rear garden, a study, utility room, generous family bathroom and guest cloakroom complete the accommodation. The property is situated on a generous plot consisting beautifully maintained gardens, a pond and a large decking area ideal for al fresco entertaining. LOCATION Ottershaw is a village in the Runnymede district of Surrey, England about 20 miles to the south-west of London. It is part of the Foxhills ward. -

Beating the Bounds
BEATING THE BOUNDS Iain Wakeford © 2014 ou might be forgiven, if you have looked at the previous pages on this site, in thinking that only Woking and Chertsey were recorded in this area before the writing of the Domesday Book. But Byfleet was recorded in 727 - fleot or fleet being an old Y name for a stream (its location being ‘by [the] fleet’) - with Pyrford following in 956 and Send in about 960-2. Crockford may refer to ‘crocks’ of pottery, being deposited near the ford as some offering to the ancient river gods! Nearby the ‘herestreat’ (a military road), may mark the route of a Roman Road (mentioned in my article on the Roman period). orsell isn’t recorded until the 13th The 7th century bounds of Chertsey Abbey The later 9th century bounds of Chertsey, century, but a number of other ‘minor’ record many local places for the first time recorded in King Alfred’s Charter of about 889, H place names do make their way into including Weybridge, Woburn and Crockford at are slightly different and mention for the first the records before the Norman Conquest, Addlestone, as well as Mimbridge (bridge near time Wintersells (Wyntredeshulle), Fullbrook including Egley (or Egceanlea – Ecga’s Clearing) the field of mint) and Sythwood (possibly (fule brok – or foul brook) and Durnford – as in the road between Woking & Mayford, ‘scythe shaped wood’) at Horsell. (derneforde) – the latter apparently meaning a recorded in about 1005 (if the 12th century ‘secret’ or hidden ford. records of Eynsham Abbey in Oxfordshire are to Of course the present day Sythwood on Goldsworth Park is just a modern Wintersells was at one time a farm off Oyster be believed). -

Surrey. Addlestone
DIRECTORY.] SURREY. ADDLESTONE. 19 St. Augustine's (infants), Albert road, erected in 1882, for Chapel Park (infants), built in 1896, for 78 children; average 100 children: average attendance, 45; Mrs. Martha Bell, attendance, 67; Miss Adela Welbom, mistress mistress & Miss Emily Bridle, assistant mistress Railway Station. Addlestone, W. H. Broadbank, station ma>ter Marked thus • receive their letters from Dclabere Richard Norman, Hazel Hat<:h, Lucy Arthur B. Fair Oaks, Liberty lane Weybridge. Ongar hill ·~fc:\Iurdie Very Rev. Thomas C.J. s~ Marked thus t receive their letters through Dennis Waiter, 50 High street George's collegf', Woburn Park West Byfleet. Desprez 1\frs. Diglis close, Chertsey road Mamham Reginald J. The Acacias, . Drewery L. Brighton villa, Brighton road Simplemarsh road ~arked thus ~receive theu letters through Duff Miss, l\Iead cottage, New Haw Martin James, Norfolk house, Station rd Ottershaw. Eales Mrs. Chapel fields, Simplemarsh rd Mayo Mrs. Helens burgh, Hare hill (let- PRIVA.TB RESIDENTg. Edwards George Musgrave, Th~ Cedars, ters through Chertsey) • Adams Aaron, Round ho. Weybridge rd Crookford Park road :\Iayor Gordon, Burcott, New Haw Adams Charles J. The Limes, Crockford. Elliott Miss. Newholme, Wobum hill ~filne John, Park house, Addlestone park Park road Elsley Fredk. Wm. Brendon, Eastworth rd Milsome Mrs. St. Ann's lodge, Church road Alien Herbert, 21 Green lane Ethcridge Regimld D. The Sycamores, t.Minshall Thomas Hcrbert, Great Grove, Alln'lan George, Mayfield av. Woodham rd Simplemarsh road Ottershaw road Arnold Edward Hicks, Southam, Addle- •Farrell Rev. John Reginald c.J. St.. MitchellJames, 19 Green lane stone park George's college, \Voburn Park Mocatta. -

HOUSING the COMMUTERS of the LATE 1930’S Iain Wakeford 2016
HOUSING THE COMMUTERS OF THE LATE 1930’s Iain Wakeford 2016 The developers of Cavendish Road benefitted from the council extending the surface water sewer in the Goldsworth area - as no doubt did those of The Dell. e have seen in previous articles how away from any pressure of future in the early and mid 1930’s local redevelopment)! builders such as Evelyn Ricks built W By 1937 the fledgling Wych Hill Way had numerous little estates on former farmland in started to be built (with just four houses listed both Westfield and Horsell, and how Thomas in the Woking Street Directory of that year), Higgs developed his estate on the land once although other houses had been given planning occupied by Woodham Hall. With the permission. One of the developers was Mr R.O. electrification of the railway through Woking in Garrard, who in September 1937 offered to 1936-7 the incentives for developers to find donate a strip of land at his ‘Turnoak Estate’ to more local land for housing increased, and soon the council if they extended the surface water little nurseries and farmers close to town were sewer to take water from his land. The council receiving offers they couldn’t refuse. accepted, possibly releasing the flood-gates (if The Jackman family had started out as farmers you will pardon the pun) on other developers in the St Johns area, but by the end of the 18th requesting similar help to drain their land. century had turned their fields into nursery- One of these was a company called Banstead grounds. -

Runnymede Borough Council Electoral Review Warding Pattern
Runnymede Borough Council Electoral Review Warding Pattern Proposal 1 Contents Introduction .................................................................................................................................. 3 Electoral Cycle & Council Size Submission ................................................................. 4 Electorate Population Size ................................................................................................ 4 Warding Proposal ....................................................................................................................... 5 Development of proposed warding pattern .................................................................. 5 The Proposed Warding Pattern ........................................................................................ 6 Addlestone North ................................................................................................................. 7 Addlestone South ................................................................................................................ 8 Chertsey Riverside .............................................................................................................. 9 Chertsey St Ann’s .............................................................................................................. 10 Egham Hythe ....................................................................................................................... 11 Egham Town ....................................................................................................................... -

The Veterinary Products Committee and Its Sub-Committees Annual Report 2007
VETERINARY PRODUCTS COMMITTEE The Veterinary Products Committee and its Sub-Committees Annual Report 2007 Veterinary Products Committee: Contacts The Veterinary Medicines Directorate (VMD) provides administrative support to the Veterinary Products Committee (VPC) and its sub-committees. Contact details are: Committee Support Team, VMD, Woodham Lane, New Haw, Addlestone, Surrey, KT15 3LS (tel: 01932 336911, email: [email protected], fax: 01932 336618), or Colin Bennett Direct line: 01932 338490 (Secretary, VPC) email: [email protected] Carol Brailsford Direct line: 01932 338492 email: [email protected] Clare Main Direct line: 01932 338491 email: [email protected] Chris Abbott Direct line: 01932 338353 email: [email protected]. Further copies of this report, and all reports referred to, are available from the Committee Support Team. Alternatively, the reports can be viewed or downloaded from the VPC’s website (www.vpc.gov.uk). If you would like to report a suspected adverse reaction involving a veterinary medicinal product, or if you want further information on the Suspected Adverse Reaction Surveillance Scheme (SARSS), please contact the SARSS team at the VMD, Woodham Lane, New Haw, Addlestone, Surrey, KT15 3LS (tel: 01932 336911, email: [email protected], fax: 01932 336618), or Denise Burge Direct line: 01932 338427 email: [email protected]. The VPC and its sub-committees annual report 2007: Index THE VETERINARY PRODUCTS COMMITTEE AND ITS SUB-COMMITTEES -

River Wey Navigation- Pyrford Lock to Weybridge Town Lock
River Wey Navigation- Pyrford Lock to Weybridge Town Lock Moderate Trail: Please be aware that the grading of this trail was set according to normal water levels and conditions. Weather and water level/conditions can change the nature of trail within a short space of time so please ensure you check both of these before heading out. Route Summary Distance: 4.8 miles Enjoy a paddle along the Wey which was one of the first Approximate Time: 1-2 Hours British rivers to be made navigable and opened to barge The time has been estimated based on you travelling 3 – 5mph traffic in 1653. It is quite unusual waterway. The Wey has (a leisurely pace using a recreational type of boat). two separate sources in two different counties. The two Type of Trail: One Way River Weys unite near the historic Tilford Oak in Surrey Waterways Travelled: River Wey Navigation The Wey and its two Navigations flow across 87 miles Type of Water: Rural and Urban River (140 km) of countryside yet drop a mere 98 feet (30 m) by the time the waterway joins the Thames at Weybridge. Portages and Locks: 2 Nearest Town: Weybridge Start: Pyrford Lock GU23 6QW Start Directions Finish: Weybridge Town Lock Map 176 Reference 069647 KT13 8XX Limited off road parking on the opposite side of the canal O.S. Sheets: Landranger No 187 – Dorking, Reigate & to the Anchor pub – Map 187 Ref 054593 GU23 6QW Crawley and No. 176 – West London The Anchor pub car park is for customers only and barrier Licence Information: A licence is required to paddle controlled. -

P7 P11 P12 P17
Issue 164 The Newsletter of the Byfleet, West Byfleet & Pyrford Residents’ Association Community spirit renewed during Thursday night clap for the NHS Carers and Support staff Each Thursday night since the start of the COVID-19 pandemic, many residents in the 3 villages including Romans Way, Pyrford (picture above) have come to their doorsteps and joined the rest of the nation in clapping and clanging saucepan lids in support of the many NHS Staff, Carers, Emergency Services and all Key Workers in their efforts to get this awful pandemic under control. A positive result has been the enhancement of the community spirit which has found many residents staying around for some time afterwards and talking to neighbours they either hadn’t spoken to for a long time or never at all!! featured ARTICLES Messages from Dealing with A message from our Competition our Churches the Coronavirus MP, Jonathan Lord Winners revealed P7 P11 P12 P17 Website: www.the-residents.org Email: [email protected] 2 Editorial Membership Report The decision to publish this issue of The Resident online and make it available to all residents on our website, may mean that you are reading about this Residents’ Association (RA) for the first time. At a time when community cohesion is Message from Surrey County Council so important, the RA membership is We’ve set up a Community Helpline on open to all people over 18 years old, 0300 200 1008 (8am – 6pm Monday Welcome to my first edition of The Resident who reside, have a place of business or to Friday, Saturday to Sunday 10am - magazine which is our first online only version have resided in Byfleet, West Byfleet 2pm) to help direct residents who need due to the Coronavirus Pandemic. -

Guildford Diocese Hearing Aid Drop-In Clinics
Guildford Diocese Hearing Aid Drop-in Clinics Venue Contact Day & Time Dates St Paul’s Church Victor Hatzfeld Wednesday 31st January 2018 Church Road 01932 560956 10.30 – 11.30 am 28th February Addlestone 28th March KT15 1SJ Eileen Tozer Day Centre Victor Hatzfeld Tuesday 2nd January 2018 Crouch Oak Lane 01932 560956 10.00 am – 12 noon 6th February Addlestone Isabel Hindle 6th March KT15 2AN [email protected] Town Centre Pastoral Team table Jan Neilsen Thursday 25th January 2018 The Wellington Centre [email protected] 10.30 am - 12.30 pm 22nd February Union Street Entrance 01252 333499 29th March Aldershot GU11 1DB Community Centre Sheila Mighall Wednesday 3rd January 2018 (Adjacent to Village Hall) [email protected] 12 noon – 12.30pm 7th February 78 High Street 01883 742003 7th March Bletchingley Bill Shillito Redhill 01883 740248 RH1 4PA Church House Guildford, 20 Alan Turing Road, Guildford, Surrey, GU2 7YF Tel: 18001 01483 790327 SMS: 07531 268 476 Fax: 01483 790333 Email: [email protected] The Guildford Diocesan Board of Finance is registered in England as a Company Limited by Guarantee (No 2255289) and a Charitable Company (No 248245) Guildford Diocese Hearing Aid Drop-in Clinics Venue Contact Day & Time Dates Parish Room Richard & Evelyn Gates Monday 1st January 2018 Holy Trinity Church [email protected] 10.00 - 11.00 am 5th February High Street 01483 893861 5th March Bramley Tuesday 2nd January 2018 Guildford 3.00 - 4.00 pm 6th February GU5 0HD 6th March The Church of the Holy Spirit Jean Davy Thursday 18th -

Addlestone Surrey
FREEHOLD DEVELOPMENT OPPORTUNITY (Subject to Planning) Land to the West of Byfleet Road New Haw Addlestone Surrey On behalf of: January 2017 Site Disposal Byfleet Road, Addlestone Development opportunity in the heart of Surrey. OVERVIEW ° Total site area of 7.85 hectares (19.39 acres). ° The majority of the surrounding land use to the north is residential, with industrial use ° Popular residential location approximately 5 and Brooklands Business Park situated to the minutes walk from Byfleet and New Haw south. train station. ° The site is currently designated as a housing ° Located approximately 3 miles form Junction reserve site in the Runnymede Local Plan 11 of the M25. 2001. ° The site is subject to two leases with vacant ° The site has been included in the within the possession due in September 2017. Strategic Land Availability Assessment (SLAA) 2016 as ‘Byfleet Road’ (Site ID - No. 51). ° Vehicular access stems directly from Byfleet Road along the eastern boundary. N Source: Bing Maps – Not to Scale Site Disposal Byfleet Road, Addlestone Development opportunity in the heart of Surrey. LOCATION / DESCRIPTION The site is located in the small village of New The site extends to approximately 7.85 hectares Haw, south of Addlestone, Surrey. It is located (19.39 acres) and is broadly rectangular in approximately 26 miles (41.8 km) south west of shape. There are five electricity pylons on site London, falling within the M25 and the Borough with overhead power lines. of Runnymede. The town is approximately 12 miles (19.3 km) south of Heathrow Airport, in The site is situated at the southern most end of close proximity to the M3 and J11 of the M25. -

Runnymede Borough Council
Air Quality Action Plan for Runnymede Borough Council In fulfillment of Part IV of the Environment Act 1995 Local Air Quality Management April 2014 EXECUTIVE SUMMARY Under the system of local Air Quality Management introduced by the Environment Act 1995, local authorities have a duty to work in pursuit of air quality objectives and work towards their achievement in a cost effective way. An area identified as unlikely to meet the objectives must be designated as an Air Quality Management Area (AQMA). After declaring an AQMA, a local authority is required to prepare a remedial Air Quality Action Plan (AQAP) to improve air quality in that area. An AQAP must provide a quantification of the source contributions to the exceedences of the relevant objectives, evidence that all available options have been considered, a plan of how the local authority will use its powers and also work in conjunction with other organisations to implement the AQAP, timescales for AQAP implementation and an impact assessment of the proposed measures. Annual review and assessment reports (summarised in Section 1.4) give an account of the current air quality in Runnymede and identify areas where national targets might not be met. As road traffic is the major source of pollution in Runnymede, the main air pollutants are nitrogen dioxide (NO2) and fine particulates. The air quality across the Borough is generally good; however, nitrogen dioxide concentrations can be of concern close to roads carrying large traffic flows or near busy congested roads in town centres. So far, Runnymede declared two AQMAs in the following areas: along the M25 (all across the Borough – declared in 2001 for both nitrogen dioxide and particulate matter (PM) and in Addlestone Town Centre (declared in 2008 for nitrogen dioxide).