These Two Workbooks Are Provided by As a Convenient Introduc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alcohols Combined 1405
ALCOHOLS COMBINED 1405 Formulas: Table 1 MW: Table 1 CAS: Table 2 RTECS: Table 2 METHOD: 1405, Issue 1 EVALUATION: PARTIAL Issue 1: 15 March 2003 OSHA : Table 2 PROPERTIES: Table 1 NIOSH: Table 2 ACGIH: Table 2 COMPOUNDS: (1) n-butyl alcohol (4) n-propyl alcohol (7) cyclohexanol (2) sec-butyl alcohol (5) allyl alcohol (8) isoamyl alcohol (3) isobutyl alcohol (6) diacetone alcohol (9) methyl isobutyl carbinol SYNONYMS: See Table 3. SAMPLING MEASUREMENT SAMPLER: SOLID SORBENT TUBE TECHNIQUE: GAS CHROMATOGRAPHY, FID (Coconut shell charcoal, 100 mg/50 mg) ANALYTE: Compounds above FLOW RATE: 0.01 to 0.2 L/min DESORPTION: 1 mL 5% 2-propanol in CS2 Compounds: (1-3 ) (4-9) VOL-MIN: 2 L 1 L INJECTION -MAX: 10 L 10 L VOLUME: 1 µL SHIPMENT: Routine TEMPERATURE -INJECTION: 220 °C SAMPLE -DETECTOR: 250 - 300 °C STABILITY: See Evaluation of Method. -COLUMN: 35 °C (7 minutes), to 60 °C at 5 °C/minute, hold 5 minutes, up to BLANKS: 2 to 10 field blanks per set 120 °C at 10 °C /minute, hold 3 minutes. CARRIER GAS: He, 4 mL/min ACCURACY COLUMN: Capillary, fused silica, 30 m x 0.32-mm RANGE STUDIED: Not studied [1, 2]. ID; 0.5 µm film polyethylene glycol, DB- wax or equivalent BIAS: Not determined CALIBRATION: Solutions of analyte in eluent (internal OVERALL standard optional) PRECISION (Ö ): Not determined rT RANGE: See EVALUATION OF METHOD. ACCURACY: Not determined ESTIMATED LOD: 1 µg each analyte per sample PRECISION: See EVALUATION OF METHOD. APPLICABILITY: This method may be used to determine two or more of the specified analytes simultaneously. -

Kinetic Modeling and Mechanistic Investigations of Transesterification of Propylene Carbonate with Methanol Over Fe-Mn Double Metal Cyanide Catalyst
Reaction Chemistry & Engineering Kinetic Modeling and Mechanistic Investigations of Transesterification of Propylene Carbonate with Methanol over Fe-Mn Double Metal Cyanide Catalyst Journal: Reaction Chemistry & Engineering Manuscript ID RE-ART-09-2019-000372.R1 Article Type: Paper Date Submitted by the 21-Oct-2019 Author: Complete List of Authors: Song, Ziwei; Yanshan University, Subramaniam, Bala; University of Kansas, Center for Environmentally Beneficial Catalysis Chaudhari, Raghunath; University of Kansas, Chemical & Petroleum Engineering Page 1 of 23 Reaction Chemistry & Engineering Kinetic Modeling and Mechanistic Investigations of Transesterification of Propylene Carbonate with Methanol over Fe-Mn Double Metal Cyanide Catalyst Ziwei Song, a,b,c Bala Subramaniam, a,b and Raghunath V. Chaudhari* a,b aCenter for Environmentally Beneficial Catalysis, University of Kansas, 1501 Wakarusa Dr. Lawrence, KS 66047, United States bDepartment of Chemical & Petroleum Engineering, University of Kansas, 1503 W 15th St. Lawrence, KS 66045, United States cDepartment of Chemical Engineering, College of Environmental and Chemical Engineering, Yanshan University, Qinhuangdao 066004, China *Corresponding author: [email protected] KEYWORDS: double metal cyanide, transesterification, dimethyl carbonate, microkinetic modeling 1 Reaction Chemistry & Engineering Page 2 of 23 Abstract Kinetic modeling of transesterification of propylene carbonate with methanol using Fe-Mn double metal cyanide catalyst has been investigated based on experimental data obtained under kinetically controlled conditions in a batch slurry reactor in the 140-200 °C range. A simple two-step power law model was found to represent the experimental data well. In addition, a detailed kinetic model based on a molecular level description of the reaction mechanism is also evaluated to provide better insight into the reaction mechanism. -

Supporting Information
Supporting Information Section 1 Components of DCM based coating strippers Table 1. Composition of Klean Strip Premium. CAS # Components Concentration 75-09-2 Dichloromethane 70.0-95.0% 67-56-1 Methanol < 5.0% 127087-87-0 Poly(oxy-1,2-ethandiyl) < 5.0% 124-38-9 Carbon dioxide < 5.0% Table 2. Composition of Klean Strip X. CAS # Components Concentration 75-09-2 Dichloromethane 30.0 – 40.0% 67-56-1 Methanol 15.0 – 26.0% 67-64-1 Acetone < 10.0% 1330-20-7 Xylene < 10.0% 108-88-3 Toluene < 10.0% 100-41-4 Ethylbenzene < 5.0% 64-17-5 Ethyl alcohol < 5.0% 67-63-0 Isopropyl alcohol < 5.0% Section 2 Sample preparation for the dwell time test As Figure S1 shows, a gasket was pasted on the conformal coating surface and a sheet of parafilm attached to the back of the printed circuit board to avoid solvent leakage dur- ing the dwell test. Figure 1. Sample preparation for the dwell time test. Section 3 Thickness measurement of coated PCBs Materials and Equipment Printed circuit boards (PCB), acrylic conformal coating, tape, Dektak stylus profiler (Bruker, Arizona, USA). Methods A piece of tape was attached on a PCB before coating. The coating was applied on the PCB using the same method as dip coating in the dwell time test. The PCB was sta- tioned and dried at room temperature for over 24 hours. The tape was then torn out to create a coating step. The PCB was fixed on the detection table using tapes. The stylus scanned from sub- strate to coated area. -

Enhanced Butanol Production by Free and Immobilized Clostridium Sp
Enhanced Butanol Production by Free and Immobilized Clostridium sp. Cells Using Butyric Acid as Co-Substrate Laili Gholizadeh This thesis comprises 30 ECTS credits and is a compulsory part in the Master of Science with a Major in Chemical Engineering – Applied Biotechnology 120 ECTS credits No. 10/2009 Title: Enhanced Butanol Production by Free and Immobilized Clostridium sp. Cells using Butyric Acid as Co-Substrate. Author: Laili Gholizadeh Baroghi (e-mail: [email protected]) Master Thesis Subject Category: Biotechnology (Bioprocess Engineering – Biofuels) University College of Borås School of Engineering SE-501 90 BORÅS Telephone: (+46) 033 435 4640 Examiner: Prof. Mohammad Taherzadeh Supervisor and Thesis Advisor: Prof. Shang–Tian Yang Supervisor Address: OSU–Ohio State University 125 Koffolt Laboratories 140 West 19th Ave. Columbus, OH 43210–1185, USA Client: Ohio State University (OSU), Chemical & Biomolecular Engineering Department Prof. Shang–Tian Yang Columbus, Ohio; USA. Date: 08–12–2009 Keywords: Bio-butanol y Acetone–Butanol–Ethanol (ABE) y ABE- fermentation y Butyric acid y Clostridium y C. acetobutylicum ATCC 824 y C. beijerinckii ATCC 55025 y C. beijerinckii BA 101 y C. beijerinckii NCIMB 8052 y Fibrous-bed Bioreactor (FBB) y Batch y Suspended cell culture y Immobilized cell system. DEDICATION I would like to dedicate this M.Sc. Thesis to my beloved Family for all their love and encouragement and for always been supportive of my choices. “I am among those who think that science has great beauty. A scientist in his laboratory is not only a technician: he is also a child placed before natural phenomena, which impress him like a fairy tale.” − Marie Curie ABSTRACT Butanol production by four different Clostridium sp. -

Propylene Carbonate Extraction for Recovery of Poly-3-Hydroxybutryate
1 Propylene Carbonate Extraction for Recovery of Poly-3-hydroxybutryate Abstract Propylene Carbonate Distillation Poly -3-hydroxybutryate (PHB) is a biopolymer produced Propylene Carbonate is an organic solvent produced as a storage molecule for energy and carbon under • Waste solution recovered after PHB by combining propylene oxide with carbon dioxide. It is poor growth conditions in certain organisms. PHB is a precipitation consisted of Propylene considered a polar aprotic solvent (no proton to donate) class of polyhydroxyalkanoates (PHAs), which have Carbonate and Ethanol Due to its characteristics (low vapor pressure, stability, similar properties to plastics derived from petroleum. PHB provides an environmentally friendly alternative to and high polarity) it is used in many applications. These include • Through distillation, both the Ethanol production of fibers and textiles, dyeing, personal care agents petroleum -based plastics due to its biodegradability and and Propylene Carbonate have been and cosmetics, and a component of some adhesives. It is also method of production. It can be produced by recovered. considered non-toxic making it more suitable to use at larger recombinant Escherichia coli, harvested from the cell, scales. and recovered. One of the major expenses of • Recovered 70% Propylene producing PHB is the extraction of the PHB from the Carbonate and nearly all Ethanol biomass. This study focused on evaluating different methods of PHB extraction for their ability to effectively • Preliminary experiments show no extract PHB from E. coli biomass and the feasibility of Propylene Carbonate Extraction Procedure Distillation apparatus to recover Propylene difference in the yield of PHB scaling up those processes. These processes provide a Carbonate and Ethanol from waste stream extracted with distilled Propylene 125 L Bioreactor way to produce PHB from growing E. -

Transcription 12.01.12
Lecture 2B • 01/12/12 We covered three different reactions for converting alcohols into leaving groups. One was to turn an alcohol into an alkyl chloride, that was using thionyl chloride. Second reaction was using tosyl chloride; the primary difference between those two reactions is one of stereochemistry. An inversion of stereochemistry does occur if you use thionyl chloride, because it does affect the carbon-oxygen bond, but because forming a tosylate does not touch the carbon-oxygen bond, only the oxygen- hydrogen bond, there’s no change in stereochemistry there. We then saw phosphorus tribromide that reacts very similarly to the thionyl chloride; you get an alkyl bromide instead, but it also has inversion of configuration. The last reaction is not a new mechanism, it is just an Sn2 reaction, it’s called the Finkelstein reaction. Really this works, in a sense, off of Le Châtelier’s principle. In solution, in theory, sodium iodide can displace bromide, but sodium bromide can displace iodide, so you can have an Sn2 reaction that goes back and forth and back and forth and back and forth. Except, sodium iodide is somewhat soluble in acetone, while sodium chloride and sodium bromide are not. So, in fact, one of the things that we get out of this as a by-product is sodium bromide, which, again, is not soluble in acetone and therefore precipitates out and is no longer part of the reaction mixture, so there’s no reverse reaction possible. Because of this solubility trick, it allows this reaction to be pulled forward, which means you can get the alkyl iodide. -

Aldehydes and Ketones
Organic Lecture Series Aldehydes And Ketones Chap 16 111 Organic Lecture Series IUPAC names • the parent alkane is the longest chain that contains the carbonyl group • for ketones, change the suffix -e to -one • number the chain to give C=O the smaller number • the IUPAC retains the common names acetone, acetophenone, and benzophenone O O O O Propanone Acetophenone Benzophenone 1-Phenyl-1-pentanone (Acetone) Commit to memory 222 Organic Lecture Series Common Names – for an aldehyde , the common name is derived from the common name of the corresponding carboxylic acid O O O O HCH HCOH CH3 CH CH3 COH Formaldehyde Formic acid Acetaldehyde Acetic acid – for a ketone , name the two alkyl or aryl groups bonded to the carbonyl carbon and add the word ketone O O O Ethyl isopropyl ketone Diethyl ketone Dicyclohexyl ketone 333 Organic Lecture Series Drawing Mechanisms • Use double-barbed arrows to indicate the flow of pairs of e - • Draw the arrow from higher e - density to lower e - density i.e. from the nucleophile to the electrophile • Removing e - density from an atom will create a formal + charge • Adding e - density to an atom will create a formal - charge • Proton transfer is fast (kinetics) and usually reversible 444 Organic Lecture Series Reaction Themes One of the most common reaction themes of a carbonyl group is addition of a nucleophile to form a tetrahedral carbonyl addition compound (intermediate). - R O Nu - + C O Nu C R R R Tetrahedral carbonyl addition compound 555 Reaction Themes Organic Lecture Series A second common theme is -

US5349077.Pdf
|||||||||||||||| USOO5349077A United States Patent (19) 11 Patent Number: 5,349,077 Doya et al. 45 Date of Patent: Sep. 20, 1994 54 PROCESS FOR PRODUCING ALKYLENE 5,003,084 3/1991 Su et al. .............................. 549/230 CARBONATES FOREIGN PATENT DOCUMENTS 75 Inventors: Masaharu Doya; Takashi Ohkawa; Yutaka Kanbara; Aksushi Okamoto; 0443758 8/1991 European Pat. Off. Kenichi Kimizuka, all of Niigata, OTHER PUBLICATIONS Japan Chemical Abstracts, vol. 82, No. 23, Jun. 9, 1975, Co 73 Assignee: Mitsubishi Gas Chemical Company, lumbus, Ohio; Sergio Fumasoni et al. Inc., Tokyo, Japan Primary Examiner-José G. Dees 21 Appl. No.: 99,461 Assistant Examiner-Joseph M. Conrad, III 22 Filed: Jul. 30, 1993 Attorney, Agent, or Firm-Wenderoth, Lind & Ponack (30) Foreign Application Priority Data (57) ABSTRACT Jul. 31, 1992 JP Japan .................................. 4-205362 A process for producing alkylene carbonates which Jul. 31, 1992 (JP) Japan .......................... ... 4-205363 comprises reacting urea and glycols described by the Jul. 31, 1992 JP Japan .......................... ... 4-205364 general formula RCH(OH) CH2OH; wherein R repre Jun. 30, 1993 JP Japan .................................. 5-161857 sents hydrogen or an alkyl group containing 1 to 4 51) Int. Cl. .............................................. CO7C 69/96 carbons, using a catalyst containing zinc, magnesium, 52 U.S. Cl. .................................... 558/260; 549/229; lead or calcium at reduced pressures. The alkylene 549/230 carbonates are produced with high yield easily using 58 Field of Search ................. 558/260; 549/229, 230 raw materials which are comparatively inexpensive with a mild reaction that does not involve explosive or 56) References Cited hazardous materials. U.S. PATENT DOCUMENTS 2,773,881 12/1956 Dunn et al. -

Silfort* SHC1200 Silfort* SHC1200
Technical Data Sheet SilFORT* SHC1200 SilFORT* SHC1200 Description SilFORT SHC1200 Hard Coat SHC1200 hard coat has been found to yield a clear mar-resistant film when applied to a suitably prepared plastic substrate. It can be applied by flow, dip or spray coating. SilFORT SHP401 Primer SHP401 air-dried primer is used as an adhesion promoter for SHC1200 hard coat on polycarbonate resin. It can be applied by flow, dip or spray coating. Key Features and Benefits Fast cure Abrasion resistance Scratch resistance Good clarity Solvent/chemical resistance SHP401 Primer No thermal cure required Improves coating adhesion Improves water resistance Page 1 of 6 *SilFORT ist ein Markenname der Momentive Performance Materials Inc. SilFORT* SHC1200 Improves ultraviolet resistance SHP401 Primer/SHC1200 Hard Coat on polycarbonate (2 - 4 micron Topcoat Thickness) Cured Film Properties Film Thickness, slow dip coat, 10-18 cm/min 2 – 4 micron withdrawal,18-20% solids at 22C Taber Abrasion, 500 cycles 500G on primed < 6.0 % Haze measured per polycarbonate (CS10F wheel) ASTM D1003. * Index of Refraction 1.4 *Humidity during coating and testing will affect final values. Typical Physical Properties Property SHC1200 Hard Coat SHP401 Primer Solids Content, % 20 ± 1 2.1 ± 0.2 Methanol, Isobutanol, 1-Methoxy-2-propanol, Diacetone Solvent Isopropanol Alcohol Flash Point, PMCC 19.4C 36.1C Density, g/cm3 0.911 0.959 pH 7.3 ± 0.2 - Viscosity, cstk @ 25°C 20 ± 3 4 - 7 Dry Film Thickness, 2.75 - 4.5 0.5 micron VOC, g/l 710 937 Patent Status Nothing contained herein shall be construed to imply the nonexistence of any relevant patents or to constitute the permission, inducement or recommendation to practice any invention covered by any patent, without authority from the owner of the patent. -

N-BUTYL ALCOHOL
Right to Know Hazardous Substance Fact Sheet Common Name: n-BUTYL ALCOHOL Synonyms: Propyl Carbinol; n-Butanol CAS Number: 71-36-3 Chemical Name: 1-Butanol RTK Substance Number: 1330 Date: November 1998 Revision: January 2008 DOT Number: UN 1120 Description and Use EMERGENCY RESPONDERS >>>> SEE BACK PAGE n-Butyl Alcohol is a colorless liquid with a strong, sweet Hazard Summary alcohol odor. It is used as a solvent for fats, waxes, shellacs, Hazard Rating NJDOH NFPA resins, gums, and varnish, in making hydraulic fluids, and in HEALTH - 2 medications for animals. FLAMMABILITY - 3 REACTIVITY - 0 f ODOR THRESHOLD = 1 to 15 ppm FLAMMABLE f Odor thresholds vary greatly. Do not rely on odor alone to POISONOUS GASES ARE PRODUCED IN FIRE determine potentially hazardous exposures. CONTAINERS MAY EXPLODE IN FIRE Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; Reasons for Citation 4=severe f n-Butyl Alcohol is on the Right to Know Hazardous f n-Butyl Alcohol can affect you when inhaled and by Substance List because it is cited by OSHA, ACGIH, DOT, passing through the skin. NIOSH, DEP, IRIS, NFPA and EPA. f Contact can irritate and burn the skin. f This chemical is on the Special Health Hazard Substance f n-Butyl Alcohol can irritate and burn the eyes with possible List. eye damage. f Inhaling n-Butyl Alcohol can irritate the nose, throat and lungs. f Exposure to n-Butyl Alcohol can cause headache, dizziness, nausea and vomiting. SEE GLOSSARY ON PAGE 5. f n-Butyl Alcohol can damage the liver, kidneys, hearing, and sense of balance. -
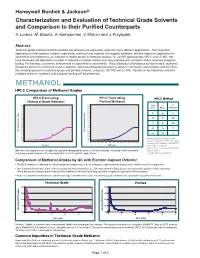
Methanol and Acetone Is in Organic Synthesis
Honeywell Burdick & Jackson® Characterization and Evaluation of Technical Grade Solvents and Comparison to their Purified Counterparts S. Lorenz, M. Bosma, A. Kemperman, V. Mohan and J. Przybytek Abstract: Technical grade solvents and their purified counterparts are separately useful for many different applications. One important application for the common solvents acetonitrile, methanol and acetone is in organic synthesis. Another important application for acetonitrile and methanol is as a diluent or mobile phase in chemical analysis, ie: UV-VIS spectroscopy, HPLC and LC-MS. We have observed and identified a number of impurities in these solvents that may interfere with synthesis and/or accurate analytical testing. For instance, a common contaminant in acetonitrile is acrylonitrile. Trace aldehydes and ketones can be found in methanol. Diacetone alcohol is commonly found in acetone. We have characterized impurity levels in commonly used solvents and will show the variability present in technical grade and purified solvents, using GC, GC-MS and LC-MS. Results on the impurities and their probable effect on synthesis and analytical testing will be presented. METHANOL HPLC Comparison of Methanol Grades HPLC Trace using HPLC Trace using HPLC Method Technical Grade Methanol Purified Methanol 0.200 0.200 Time % % 0.190 0.190 0.180 0.180 (min) Water Methanol 0.170 0.170 0.160 0.160 0.150 0.150 0 95 5 0.140 AU) 0.140 AU) ( ( 0.130 0.130 e e 20 0 100 c 0.120 c 0.120 n 0.110 n 0.110 a a b 0.100 b 0.100 r r 25 0 100 o 0.090 o 0.090 s s 0.080 0.080 -

US EPA, Inert (Other) Pesticide Ingredients in Pesticide Products
Inert Ingredients ordered by CAS Number Updated August 2004 CAS PREFIX NAME List No. 50-21-5 Lactic acid 4B 50-70-4 Sorbitol 4A 50-81-7 L- Ascorbic acid 4A 50-99-7 Dextrose 4A 51-03-6 Piperonyl butoxide 3 51-05-8 Procaine hydrochloride 3 51-55-8 Atropine 3 52-51-7 2- Bromo-2-nitro-propane-1,3-dio 3 54-21-7 Sodium salicylate 3 56-81-5 Glycerol (glycerin) 1,2,3 propanetriol 4A 56-86-0 L- Glutamic acid 3 56-95-1 Chlorhexidine diacetate 3 57-10-3 Hexadecanoic acid 4A 57-11-4 Stearic acid 4A 57-13-6 Urea 4A 57-48-7 D- Fructose 4B 57-50-1 Sugar 4A 57-55-6 Propylene glycol 4B 57-88-5 (3.beta.)- Cholest-5-en-3-ol 4B 58-08-2 1H- Purine-2,6-dione, 3,7-dihydro-1,3,7-trimethyl- 4B 58-56-0 Thiamine mononitrate 4B 58-85-5 Biotin 3 58-86-6 D- Xylose 4B 58-95-7 Vitamin E acetate 3 59-30-3 Folic acid 4B 59-40-5 N-(2- Quinoxalinyl)sulfanilide 3 59-67-6 Nicotinic acid 3 60-00-4 Ethylenediaminetetraacetic acid (EDTA) 4B 60-12-8 Benzeneethanol 3 60-29-7 Ethane, 1,1'-oxybis- 3 60-33-3 Linoleic acid 3 61-73-4 C.I. Basic Blue 9 3 62-33-9 Ethylenediaminetetraacetic acid (EDTA), calcium4B 62-54-4 Acetic acid, calcium salt 4A 63-42-3 D-(+)-Lactose 4A 63-68-3 L- Methionine 4B 64-02-8 Ethylenediaminetetraacetic acid (EDTA), tetraso4B 64-17-5 Ethanol 4B 64-18-6 Formic acid 3 64-19-7 Acetic acid 4B 64-86-8 Colchicine 3 65-85-0 Benzoic acid 4B 66-71-7 1,10- Phenanthroline 3 67-03-8 Thiamin hydrochloride 3 67-43-6 1,1,4,7,7- Diethylenetriaminepentaacetic acid 3 67-48-1 Choline chloride 4B 67-56-1 Methyl alcohol 3 67-63-0 2- Propanol 4B 67-64-1 Acetone 3 67-68-5 Dimethyl