דוקטור לפילוסופיה Doctor of Philosophy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inherited Monogenic Defects of Ceramide Metabolism Molecular
Clinica Chimica Acta 495 (2019) 457–466 Contents lists available at ScienceDirect Clinica Chimica Acta journal homepage: www.elsevier.com/locate/cca Review Inherited monogenic defects of ceramide metabolism: Molecular bases and diagnoses T ⁎⁎ Patricia Dubota,b, Frédérique Sabourdya,b, Jitka Rybovac,Jeffrey A. Medinc,d, , ⁎ Thierry Levadea,b, a Laboratoire de Biochimie Métabolique, Centre de Référence en Maladies Héréditaires du Métabolisme, Institut Fédératif de Biologie, CHU de Toulouse, Toulouse, France b INSERM UMR1037, CRCT (Cancer Research Center of Toulouse), Université Paul Sabatier, Toulouse, France c Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, USA d Department of Biochemistry, Medical College of Wisconsin, Milwaukee, WI, USA ABSTRACT Ceramides are membrane lipids implicated in the regulation of numerous biological functions. Recent evidence suggests that specific subsets of molecular species of ceramide may play distinct physiological roles. The importance of this family of molecules in vertebrates is witnessed by the deleterious consequences of genetic alterations in ceramide metabolism. This brief review summarizes the clinical presentation of human disorders due to the deficiency of enzymes involved either in the biosynthesis or the degradation of ceramides. Information on the possible underlying pathophysiological mechanisms is also provided, based on knowledge gathered from animal models of these inherited rare conditions. When appropriate, tools for chemical and molecular diagnosis of these disorders and therapeutic options are also presented. 1. Introduction/foreword relationships that are relevant for unraveling the biological role of these genes and gene products in humans. Studies on ceramides and sphingolipid metabolism have attracted a lot of attention recently. This is largely related to the multiplicity of 2. -

Enhancing Skin Health: by Oral Administration of Natural Compounds and Minerals with Implications to the Dermal Microbiome
Review Enhancing Skin Health: By Oral Administration of Natural Compounds and Minerals with Implications to the Dermal Microbiome David L. Vollmer 1, Virginia A. West 1 and Edwin D. Lephart 2,* 1 4Life Research, Scientific Research Division, Sandy, Utah 84070, USA; [email protected] (D.L.V.); [email protected] (V.A.W) 2 Department of Physiology, Developmental Biology and The Neuroscience Center, Brigham Young University, Provo, Utah 84602, USA * Correspondence: [email protected]; Tel.: +1-801-422-2006 Received: 23 August 2018; Accepted: 1 October 2018; Published: 7 October 2018 Abstract: The history of cosmetics goes back to early Egyptian times for hygiene and health benefits while the history of topical applications that provide a medicinal treatment to combat dermal aging is relatively new. For example, the term cosmeceutical was first coined by Albert Kligman in 1984 to describe topical products that afford both cosmetic and therapeutic benefits. However, beauty comes from the inside. Therefore, for some time scientists have considered how nutrition reflects healthy skin and the aging process. The more recent link between nutrition and skin aging began in earnest around the year 2000 with the demonstrated increase in peer-reviewed scientific journal reports on this topic that included biochemical and molecular mechanisms of action. Thus, the application of: (a) topical administration from outside into the skin and (b) inside by oral consumption of nutritionals to the outer skin layers is now common place and many journal reports exhibit significant improvement for both on a variety of dermal parameters. Therefore, this review covers, where applicable, the history, chemical structure, and sources such as biological and biomedical properties in the skin along with animal and clinical data on the oral applications of: (a) collagen, (b) ceramide, (c) β-carotene, (d) astaxanthin, (e) coenzyme Q10, (f) colostrum, (g) zinc, and (h) selenium in their mode of action or function in improving dermal health by various quantified endpoints. -

0.5) in Stat3∆/∆ Compared with Stat3flox/Flox
Supplemental Table 2 Genes down-regulated (<0.5) in Stat3∆/∆ compared with Stat3flox/flox Probe ID Gene Symbol Gene Description Entrez gene ID 1460599_at Ermp1 endoplasmic reticulum metallopeptidase 1 226090 1460463_at H60c histocompatibility 60c 670558 1460431_at Gcnt1 glucosaminyl (N-acetyl) transferase 1, core 2 14537 1459979_x_at Zfp68 zinc finger protein 68 24135 1459747_at --- --- --- 1459608_at --- --- --- 1459168_at --- --- --- 1458718_at --- --- --- 1458618_at --- --- --- 1458466_at Ctsa cathepsin A 19025 1458345_s_at Colec11 collectin sub-family member 11 71693 1458046_at --- --- --- 1457769_at H60a histocompatibility 60a 15101 1457680_a_at Tmem69 transmembrane protein 69 230657 1457644_s_at Cxcl1 chemokine (C-X-C motif) ligand 1 14825 1457639_at Atp6v1h ATPase, H+ transporting, lysosomal V1 subunit H 108664 1457260_at 5730409E04Rik RIKEN cDNA 5730409E04Rik gene 230757 1457070_at --- --- --- 1456893_at --- --- --- 1456823_at Gm70 predicted gene 70 210762 1456671_at Tbrg3 transforming growth factor beta regulated gene 3 21378 1456211_at Nlrp10 NLR family, pyrin domain containing 10 244202 1455881_at Ier5l immediate early response 5-like 72500 1455576_at Rinl Ras and Rab interactor-like 320435 1455304_at Unc13c unc-13 homolog C (C. elegans) 208898 1455241_at BC037703 cDNA sequence BC037703 242125 1454866_s_at Clic6 chloride intracellular channel 6 209195 1453906_at Med13l mediator complex subunit 13-like 76199 1453522_at 6530401N04Rik RIKEN cDNA 6530401N04 gene 328092 1453354_at Gm11602 predicted gene 11602 100380944 1453234_at -

Supplementary Table 1
Supplementary Table 1. 492 genes are unique to 0 h post-heat timepoint. The name, p-value, fold change, location and family of each gene are indicated. Genes were filtered for an absolute value log2 ration 1.5 and a significance value of p ≤ 0.05. Symbol p-value Log Gene Name Location Family Ratio ABCA13 1.87E-02 3.292 ATP-binding cassette, sub-family unknown transporter A (ABC1), member 13 ABCB1 1.93E-02 −1.819 ATP-binding cassette, sub-family Plasma transporter B (MDR/TAP), member 1 Membrane ABCC3 2.83E-02 2.016 ATP-binding cassette, sub-family Plasma transporter C (CFTR/MRP), member 3 Membrane ABHD6 7.79E-03 −2.717 abhydrolase domain containing 6 Cytoplasm enzyme ACAT1 4.10E-02 3.009 acetyl-CoA acetyltransferase 1 Cytoplasm enzyme ACBD4 2.66E-03 1.722 acyl-CoA binding domain unknown other containing 4 ACSL5 1.86E-02 −2.876 acyl-CoA synthetase long-chain Cytoplasm enzyme family member 5 ADAM23 3.33E-02 −3.008 ADAM metallopeptidase domain Plasma peptidase 23 Membrane ADAM29 5.58E-03 3.463 ADAM metallopeptidase domain Plasma peptidase 29 Membrane ADAMTS17 2.67E-04 3.051 ADAM metallopeptidase with Extracellular other thrombospondin type 1 motif, 17 Space ADCYAP1R1 1.20E-02 1.848 adenylate cyclase activating Plasma G-protein polypeptide 1 (pituitary) receptor Membrane coupled type I receptor ADH6 (includes 4.02E-02 −1.845 alcohol dehydrogenase 6 (class Cytoplasm enzyme EG:130) V) AHSA2 1.54E-04 −1.6 AHA1, activator of heat shock unknown other 90kDa protein ATPase homolog 2 (yeast) AK5 3.32E-02 1.658 adenylate kinase 5 Cytoplasm kinase AK7 -

The Role of Ceramides in Cigarette Smoke-Induced Alveolar Cell Death
THE ROLE OF CERAMIDES IN CIGARETTE SMOKE-INDUCED ALVEOLAR CELL DEATH Krzysztof Kamocki Submitted to the faculty of the University Graduate School in partial fulfillment of the requirements for the degree Doctor of Philosophy in the Department of Biochemistry and Molecular Biology Indiana University November 2012 Accepted by the Faculty of Indiana University, in partial fulfillment of the requirements for the degree of Doctor of Philosophy. _______________________________ Irina Petrache, M.D., Chair _______________________________ Susan Gunst, Ph.D. Doctoral Committee _______________________________ Laurence Quilliam, Ph.D. August, 22nd, 2012 _______________________________ Simon Atkinson, Ph.D. ii Dedication I dedicate my thesis to my wife, Malgorzata Maria Kamocka. iii Acknowledgements I would like to thank Dr. Irina Petrache for being my mentor during my graduate program. Dr. Petrache is not only an exceptional scientist, but also an excellent teacher. Thank you for your advice and guidelines during my scientific journey. Thank you for support and for teaching me how to think critically, for teaching me all of the aspects, which are important for a successful scientist. Thank you for your investment in me, both in funding and time you spent. In your laboratory I had an opportunity not only to learn how to design, perform experiments, and analyzed data, but also I was feeling unrestrained due to freedom for scientific exploration you offered. I would also like to thank the other members of my research committee: Dr. Susan Gunst, Dr. Lawrence Quilliam, and Dr. Simon Atkinson. Thank you all for your time, advice, constructive criticism and support. Your guidance during my graduate study was extremely helpful. -

Biological Functions of Sphingomyelin Synthase Related Protein and Ceramide Synthase 4 Investigated with Transgenic Mouse Mutants
Biological functions of Sphingomyelin synthase related protein and Ceramide synthase 4 investigated with transgenic mouse mutants Dissertation Zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn vorgelegt von Andreas Bickert aus Neuwied Bonn, 2016 Angefertigt mit Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn Erstgutachter: Prof. Dr. Klaus Willecke Zweitgutachter: Prof. Dr. Michael Hoch Tag der Promotion: 25.10.2016 Erscheinungsjahr: 2017 Table of Contents Table of Contents 1 Introduction.................................................................................................. 1 1.1 Biological lipids ............................................................................................ 1 1.2 Eucaryotic membranes ................................................................................ 3 1.3 Sphingolipids ............................................................................................... 5 1.3.1 Sphingolipid metabolic pathway ................................................................. 6 1.3.1.1 De novo sphingolipid biosynthesis .......................................................... 8 1.3.1.2 The ceramide transfer protein ................................................................. 8 1.3.1.3 Biosynthesis of complex sphingolipids .................................................... 9 1.3.1.4 Sphingolipid degradation and the salvage -

Disorders of Sphingolipid Synthesis, Sphingolipidoses, Niemann-Pick Disease Type C and Neuronal Ceroid Lipofuscinoses
551 38 Disorders of Sphingolipid Synthesis, Sphingolipidoses, Niemann-Pick Disease Type C and Neuronal Ceroid Lipofuscinoses Marie T. Vanier, Catherine Caillaud, Thierry Levade 38.1 Disorders of Sphingolipid Synthesis – 553 38.2 Sphingolipidoses – 556 38.3 Niemann-Pick Disease Type C – 566 38.4 Neuronal Ceroid Lipofuscinoses – 568 References – 571 J.-M. Saudubray et al. (Eds.), Inborn Metabolic Diseases, DOI 10.1007/978-3-662-49771-5_ 38 , © Springer-Verlag Berlin Heidelberg 2016 552 Chapter 38 · Disor ders of Sphingolipid Synthesis, Sphingolipidoses, Niemann-Pick Disease Type C and Neuronal Ceroid Lipofuscinoses O C 22:0 (Fatty acid) Ganglio- series a series b HN OH Sphingosine (Sphingoid base) OH βββ β βββ β Typical Ceramide (Cer) -Cer -Cer GD1a GT1b Glc ββββ βββ β Gal -Cer -Cer Globo-series GalNAc GM1a GD1b Neu5Ac βαββ -Cer Gb4 ββ β ββ β -Cer -Cer αβ β -Cer GM2 GD2 Sphingomyelin Pcholine-Cer Gb3 B4GALNT1 [SPG46] [SPG26] β β β ββ ββ CERS1-6 GBA2 -Cer -Cer ST3GAL5 -Cer -Cer So1P So Cer GM3 GD3 GlcCer - LacCer UDP-Glc UDP Gal CMP -Neu5Ac - UDP Gal PAPS Glycosphingolipids GalCer Sulfatide ββ Dihydro -Cer -Cer SO 4 Golgi Ceramide apparatus 2-OH- 2-OH-FA Acyl-CoA FA2H CERS1-6 [SPG35] CYP4F22 ω-OH- ω-OH- FA Acyl-CoA ULCFA ULCFA-CoA ULCFA GM1, GM2, GM3: monosialo- Sphinganine gangliosides Endoplasmic GD3, GD2, GD1a, GD1b: disialo-gangliosides reticulum KetoSphinganine GT1b: trisialoganglioside SPTLC1/2 [HSAN1] N-acetyl-neuraminic acid: sialic acid found in normal human cells Palmitoyl-CoA Deoxy-sphinganine + Serine +Ala or Gly Deoxymethylsphinganine 38 . Fig. 38.1 Schematic representation of the structure of the main sphingolipids , and their biosynthetic pathways. -

Novel Intrinsic and Extrinsic Approaches to Selectively Regulate
Novel Intrinsic and Extrinsic Approaches to Selectively Regulate Glycosphingolipid Metabolism by Mustafa Ali Kamani A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Graduate Department of Biochemistry University of Toronto © Copyright by Mustafa Ali Kamani 2013. Novel Intrinsic and Extrinsic Approaches to Selectively Regulate Glycosphingolipid Metabolism. Doctor of Philosophy, 2013. Mustafa Ali Kamani Department of Biochemistry University of Toronto Abstract Glycosphingolipid (GSL) metabolism is a complex process involving proteins and enzymes at distinct locations within the cell. Mammalian GSLs are typically based on glucose or galactose, forming glucosylceramide (GlcCer) and galactosylceramide (GalCer). Most GSLs are derived from GlcCer, which is synthesized on the cytosolic leaflet of the Golgi, while all subsequent GSLs are synthesized on the lumenal side. We have utilized both pharamacological and genetic manipulation approaches to selectively regulate GSL metabolism and better understand its mechanistic details. We have developed analogues of GlcCer and GalCer by substituting the fatty acid moiety with an adamanatane frame. The resulting adamantylGSLs are more water- soluble than their natural counterparts. These analogues selectively interfere with GSL metabolism at particular points within the metabolic pathway. At 40 µM, adaGlcCer prevents synthesis of all GSLs downstream of GlcCer, while also elevating GlcCer levels, by inhibiting lactosylceramide (LacCer) synthase and glucocerebrosidase, respectively. AdaGalCer specifically reduces synthesis of globotriaosylceramide (Gb3) and downstream globo-series GSLs. AdaGalCer also increases Gaucher disease N370S glucocerebrosidase expression, lysosomal localization and activity. AdaGSLs, therefore, have potential as novel therapeutic ii agents in diseases characterized by GSL anomalies and as tools to study the effects of GSL modulation. -

(12) Patent Application Publication (10) Pub. No.: US 2009/0269772 A1 Califano Et Al
US 20090269772A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0269772 A1 Califano et al. (43) Pub. Date: Oct. 29, 2009 (54) SYSTEMS AND METHODS FOR Publication Classification IDENTIFYING COMBINATIONS OF (51) Int. Cl. COMPOUNDS OF THERAPEUTIC INTEREST CI2O I/68 (2006.01) CI2O 1/02 (2006.01) (76) Inventors: Andrea Califano, New York, NY G06N 5/02 (2006.01) (US); Riccardo Dalla-Favera, New (52) U.S. Cl. ........... 435/6: 435/29: 706/54; 707/E17.014 York, NY (US); Owen A. (57) ABSTRACT O'Connor, New York, NY (US) Systems, methods, and apparatus for searching for a combi nation of compounds of therapeutic interest are provided. Correspondence Address: Cell-based assays are performed, each cell-based assay JONES DAY exposing a different sample of cells to a different compound 222 EAST 41ST ST in a plurality of compounds. From the cell-based assays, a NEW YORK, NY 10017 (US) Subset of the tested compounds is selected. For each respec tive compound in the Subset, a molecular abundance profile from cells exposed to the respective compound is measured. (21) Appl. No.: 12/432,579 Targets of transcription factors and post-translational modu lators of transcription factor activity are inferred from the (22) Filed: Apr. 29, 2009 molecular abundance profile data using information theoretic measures. This data is used to construct an interaction net Related U.S. Application Data work. Variances in edges in the interaction network are used to determine the drug activity profile of compounds in the (60) Provisional application No. 61/048.875, filed on Apr. -

The Concise Guide to PHARMACOLOGY 2015/16: Enzymes
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. British Journal of Pharmacology (2015) 172, 6024–6109 THE CONCISE GUIDE TO PHARMACOLOGY 2015/16: Enzymes Stephen PH Alexander1, Doriano Fabbro2, Eamonn Kelly3, Neil Marrion3, John A Peters4, Helen E Benson5, Elena Faccenda5, Adam J Pawson5, Joanna L Sharman5, Christopher Southan5, Jamie A Davies5 and CGTP Collaborators 1 School of Biomedical Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK, 2 PIQUR Therapeutics, Basel 4057, Switzerland, 3 School of Physiology and Pharmacology, University of Bristol, Bristol, BS8 1TD, UK, 4 Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK, 5 Centre for Integrative Physiology, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2015/16 provides concise overviews of the key properties of over 1750 human drug targets with their pharmacology, plus links to an open access knowledgebase of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. The full contents can be found at http://onlinelibrary.wiley.com/doi/ 10.1111/bph.13354/full. G protein-coupled receptors are one of the eight major pharmacological targets into which the Guide is divided, with the others being: G protein-coupled receptors, ligand-gated ion channels, voltage-gated ion channels, other ion channels, nuclear hormone receptors, catalytic receptors and transporters. These are presented with nomenclature guidance and summary information on the best available pharmacological tools, alongside key references and suggestions for further reading. The Concise Guide is published in landscape format in order to facilitate comparison of related targets. -

A Meta-Analysis of the Effects of High-LET Ionizing Radiations in Human Gene Expression
Supplementary Materials A Meta-Analysis of the Effects of High-LET Ionizing Radiations in Human Gene Expression Table S1. Statistically significant DEGs (Adj. p-value < 0.01) derived from meta-analysis for samples irradiated with high doses of HZE particles, collected 6-24 h post-IR not common with any other meta- analysis group. This meta-analysis group consists of 3 DEG lists obtained from DGEA, using a total of 11 control and 11 irradiated samples [Data Series: E-MTAB-5761 and E-MTAB-5754]. Ensembl ID Gene Symbol Gene Description Up-Regulated Genes ↑ (2425) ENSG00000000938 FGR FGR proto-oncogene, Src family tyrosine kinase ENSG00000001036 FUCA2 alpha-L-fucosidase 2 ENSG00000001084 GCLC glutamate-cysteine ligase catalytic subunit ENSG00000001631 KRIT1 KRIT1 ankyrin repeat containing ENSG00000002079 MYH16 myosin heavy chain 16 pseudogene ENSG00000002587 HS3ST1 heparan sulfate-glucosamine 3-sulfotransferase 1 ENSG00000003056 M6PR mannose-6-phosphate receptor, cation dependent ENSG00000004059 ARF5 ADP ribosylation factor 5 ENSG00000004777 ARHGAP33 Rho GTPase activating protein 33 ENSG00000004799 PDK4 pyruvate dehydrogenase kinase 4 ENSG00000004848 ARX aristaless related homeobox ENSG00000005022 SLC25A5 solute carrier family 25 member 5 ENSG00000005108 THSD7A thrombospondin type 1 domain containing 7A ENSG00000005194 CIAPIN1 cytokine induced apoptosis inhibitor 1 ENSG00000005381 MPO myeloperoxidase ENSG00000005486 RHBDD2 rhomboid domain containing 2 ENSG00000005884 ITGA3 integrin subunit alpha 3 ENSG00000006016 CRLF1 cytokine receptor like -
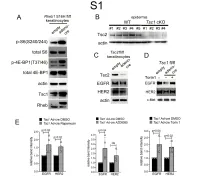
Downloaded from the Mouse Lysosome Gene Database, Mlgdb
1 Supplemental Figure Legends 2 3 Supplemental Figure S1: Epidermal-specific mTORC1 gain-of-function models show 4 increased mTORC1 activation and down-regulate EGFR and HER2 protein expression in a 5 mTORC1-sensitive manner. (A) Immunoblotting of Rheb1 S16H flox/flox keratinocyte cultures 6 infected with empty or adenoviral cre recombinase for markers of mTORC1 (p-S6, p-4E-BP1) 7 activity. (B) Tsc1 cKO epidermal lysates also show decreased expression of TSC2 by 8 immunoblotting of the same experiment as in Figure 2A. (C) Immunoblotting of Tsc2 flox/flox 9 keratinocyte cultures infected with empty or adenoviral cre recombinase showing decreased EGFR 10 and HER2 protein expression. (D) Expression of EGFR and HER2 was decreased in Tsc1 cre 11 keratinocytes compared to empty controls, and up-regulated in response to Torin1 (1µM, 24 hrs), 12 by immunoblot analyses. Immunoblots are contemporaneous and parallel from the same biological 13 replicate and represent the same experiment as depicted in Figure 7B. (E) Densitometry 14 quantification of representative immunoblot experiments shown in Figures 2E and S1D (r≥3; error 15 bars represent STDEV; p-values by Student’s T-test). 16 17 18 19 20 21 22 23 Supplemental Figure S2: EGFR and HER2 transcription are unchanged with epidermal/ 24 keratinocyte Tsc1 or Rptor loss. Egfr and Her2 mRNA levels in (A) Tsc1 cKO epidermal lysates, 25 (B) Tsc1 cKO keratinocyte lysates and(C) Tsc1 cre keratinocyte lysates are minimally altered 26 compared to their respective controls. (r≥3; error bars represent STDEV; p-values by Student’s T- 27 test).