In Vitro Preformed Biofilms of Bacillus Safensis Inhibit the Adhesion And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Food Poisoning Toxins of Bacillus Cereus
toxins Review The Food Poisoning Toxins of Bacillus cereus Richard Dietrich 1,†, Nadja Jessberger 1,*,†, Monika Ehling-Schulz 2 , Erwin Märtlbauer 1 and Per Einar Granum 3 1 Department of Veterinary Sciences, Faculty of Veterinary Medicine, Ludwig Maximilian University of Munich, Schönleutnerstr. 8, 85764 Oberschleißheim, Germany; [email protected] (R.D.); [email protected] (E.M.) 2 Department of Pathobiology, Functional Microbiology, Institute of Microbiology, University of Veterinary Medicine Vienna, 1210 Vienna, Austria; [email protected] 3 Department of Food Safety and Infection Biology, Faculty of Veterinary Medicine, Norwegian University of Life Sciences, P.O. Box 5003 NMBU, 1432 Ås, Norway; [email protected] * Correspondence: [email protected] † These authors have contributed equally to this work. Abstract: Bacillus cereus is a ubiquitous soil bacterium responsible for two types of food-associated gastrointestinal diseases. While the emetic type, a food intoxication, manifests in nausea and vomiting, food infections with enteropathogenic strains cause diarrhea and abdominal pain. Causative toxins are the cyclic dodecadepsipeptide cereulide, and the proteinaceous enterotoxins hemolysin BL (Hbl), nonhemolytic enterotoxin (Nhe) and cytotoxin K (CytK), respectively. This review covers the current knowledge on distribution and genetic organization of the toxin genes, as well as mechanisms of enterotoxin gene regulation and toxin secretion. In this context, the exceptionally high variability of toxin production between single strains is highlighted. In addition, the mode of action of the pore-forming enterotoxins and their effect on target cells is described in detail. The main focus of this review are the two tripartite enterotoxin complexes Hbl and Nhe, but the latest findings on cereulide and CytK are also presented, as well as methods for toxin detection, and the contribution of further putative virulence factors to the diarrheal disease. -

The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio In
microorganisms Review The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease Spase Stojanov 1,2, Aleš Berlec 1,2 and Borut Štrukelj 1,2,* 1 Faculty of Pharmacy, University of Ljubljana, SI-1000 Ljubljana, Slovenia; [email protected] (S.S.); [email protected] (A.B.) 2 Department of Biotechnology, Jožef Stefan Institute, SI-1000 Ljubljana, Slovenia * Correspondence: borut.strukelj@ffa.uni-lj.si Received: 16 September 2020; Accepted: 31 October 2020; Published: 1 November 2020 Abstract: The two most important bacterial phyla in the gastrointestinal tract, Firmicutes and Bacteroidetes, have gained much attention in recent years. The Firmicutes/Bacteroidetes (F/B) ratio is widely accepted to have an important influence in maintaining normal intestinal homeostasis. Increased or decreased F/B ratio is regarded as dysbiosis, whereby the former is usually observed with obesity, and the latter with inflammatory bowel disease (IBD). Probiotics as live microorganisms can confer health benefits to the host when administered in adequate amounts. There is considerable evidence of their nutritional and immunosuppressive properties including reports that elucidate the association of probiotics with the F/B ratio, obesity, and IBD. Orally administered probiotics can contribute to the restoration of dysbiotic microbiota and to the prevention of obesity or IBD. However, as the effects of different probiotics on the F/B ratio differ, selecting the appropriate species or mixture is crucial. The most commonly tested probiotics for modifying the F/B ratio and treating obesity and IBD are from the genus Lactobacillus. In this paper, we review the effects of probiotics on the F/B ratio that lead to weight loss or immunosuppression. -

Bacillus Safensis FO-36B and Bacillus Pumilus SAFR-032: a Whole Genome Comparison of Two Spacecraft Assembly Facility Isolates
bioRxiv preprint doi: https://doi.org/10.1101/283937; this version posted April 24, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Bacillus safensis FO-36b and Bacillus pumilus SAFR-032: A Whole Genome 2 Comparison of Two Spacecraft Assembly Facility Isolates 3 Madhan R Tirumalai1, Victor G. Stepanov1, Andrea Wünsche1, Saied Montazari1, 4 Racquel O. Gonzalez1, Kasturi Venkateswaran2, George. E. Fox1§ 5 1Department of Biology and Biochemistry, University of Houston, Houston, TX, 77204-5001. 6 2 Biotechnology & Planetary Protection Group, NASA Jet Propulsion Laboratories, California 7 Institute of Technology, Pasadena, CA, 91109. 8 9 §Corresponding author: 10 Dr. George E. Fox 11 Dept. Biology & Biochemistry 12 University of Houston, Houston, TX 77204-5001 13 713-743-8363; 713-743-8351 (FAX); email: [email protected] 14 15 Email addresses: 16 MRT: [email protected] 17 VGS: [email protected] 18 AW: [email protected] 19 SM: [email protected] 20 ROG: [email protected] 21 KV: [email protected] 22 GEF: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/283937; this version posted April 24, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 23 Keywords: Planetary protection, Bacillus endospores, extreme radiation resistance, peroxide 24 resistance, genome comparison, phage insertions 25 26 Background 27 Microbial persistence in built environments such as spacecraft cleanroom facilities [1-3] is often 28 characterized by their unusual resistances to different physical and chemical factors [1, 4-7]. -

Novel Bacterial Strains Pseudomonas Sp. and Bacillus Sp. Isolated from Petroleum Oil Contaminated Soils for Degradation of Flourene and Phenanthrene
Pollution, 5(3): 657-669, Summer 2019 DOI: 10.22059/poll.2019.274084.571 Print ISSN: 2383-451X Online ISSN: 2383-4501 Web Page: https://jpoll.ut.ac.ir, Email: [email protected] Novel Bacterial Strains Pseudomonas sp. and Bacillus sp. Isolated from Petroleum Oil Contaminated Soils for Degradation of Flourene and Phenanthrene Bharti, V., Gupta, B. and Kaur, J.* UIET, Biotechnology Branch, Panjab University, Chandigarh, P.O. Box 160014, India. Received: 16.01.2019 Accepted: 10.04.2019 ABSTRACT: Flourene and phenanthrene are organic compounds with high hydrophobicity and toxicity. Being recalcitrant in nature they are accumulating in the environment at an alarming concentration, posing serious threat to living beings. Thus in the present study, microorganisms were screened for their ability to degrade these contaminants at high concentrations in least period of time. Two out of fifteen isolates screened showed growth in basal medium containing 25 mg/l of fluorene/phenanthrene as the only carbon source. These selected isolates were acclimatised with step wise increased concentrations of flourene/phenanthrene for 165 days in basal medium. The acclimatised strains were identified and characterised on the basis of their morphological and biochemical characteristics and 16S rRNA gene sequence analysis. Results showed close relatedness of the isolates to Pseudomonas aeruginosa sp. and Bacillus safensis sp. Biodegradation studies carried out with these acclimatised strains at optimum conditions (pH 7 and temperature 30°C) showed 62.44% degradation of fluorene and 54.21% of phenanthrene in 10 days by Pseudomonas sp. VB92, whereas, Bacillus sp. JK17 degraded 43.64% of fluorene and 59.91% of phenanthrene in 12 days, at an initial concentration of 200 mg/l, as determined by HPTLC. -

Characterisation of Bacteria Isolated from the Stingless Bee, Heterotrigona Itama, Honey, Bee Bread and Propolis
Characterisation of bacteria isolated from the stingless bee, Heterotrigona itama, honey, bee bread and propolis Mohamad Syazwan Ngalimat1,2,*, Raja Noor Zaliha Raja Abd. Rahman1,2, Mohd Termizi Yusof2, Amir Syahir1,3 and Suriana Sabri1,2,* 1 Enzyme and Microbial Technology Research Center, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, Serdang, Selangor, Malaysia 2 Department of Microbiology, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, Serdang, Selangor, Malaysia 3 Department of Biochemistry, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, Serdang, Selangor, Malaysia * These authors contributed equally to this work. ABSTRACT Bacteria are present in stingless bee nest products. However, detailed information on their characteristics is scarce. Thus, this study aims to investigate the characteristics of bacterial species isolated from Malaysian stingless bee, Heterotrigona itama, nest products. Honey, bee bread and propolis were collected aseptically from four geographical localities of Malaysia. Total plate count (TPC), bacterial identification, phenotypic profile and enzymatic and antibacterial activities were studied. The results indicated that the number of TPC varies from one location to another. A total of 41 different bacterial isolates from the phyla Firmicutes, Proteobacteria and Actinobacteria were identified. Bacillus species were the major bacteria found. Therein, Bacillus cereus was the most frequently isolated species followed by -

Unraveling the Underlying Heavy Metal Detoxification Mechanisms Of
microorganisms Review Unraveling the Underlying Heavy Metal Detoxification Mechanisms of Bacillus Species Badriyah Shadid Alotaibi 1, Maryam Khan 2 and Saba Shamim 2,* 1 Department of Pharmaceutical Sciences, College of Pharmacy, Princess Nourah Bint Abdulrahman University, Riyadh 11671, Saudi Arabia; [email protected] 2 Institute of Molecular Biology and Biotechnology (IMBB), Defence Road Campus, The University of Lahore, Lahore 55150, Pakistan; [email protected] * Correspondence: [email protected] Abstract: The rise of anthropogenic activities has resulted in the increasing release of various contaminants into the environment, jeopardizing fragile ecosystems in the process. Heavy metals are one of the major pollutants that contribute to the escalating problem of environmental pollution, being primarily introduced in sensitive ecological habitats through industrial effluents, wastewater, as well as sewage of various industries. Where heavy metals like zinc, copper, manganese, and nickel serve key roles in regulating different biological processes in living systems, many heavy metals can be toxic even at low concentrations, such as mercury, arsenic, cadmium, chromium, and lead, and can accumulate in intricate food chains resulting in health concerns. Over the years, many physical and chemical methods of heavy metal removal have essentially been investigated, but their disadvantages like the generation of chemical waste, complex downstream processing, and the uneconomical cost of both methods, have rendered them inefficient,. Since then, microbial bioremediation, particularly the Citation: Alotaibi, B.S.; Khan, M.; use of bacteria, has gained attention due to the feasibility and efficiency of using them in removing Shamim, S. Unraveling the heavy metals from contaminated environments. Bacteria have several methods of processing heavy Underlying Heavy Metal metals through general resistance mechanisms, biosorption, adsorption, and efflux mechanisms. -

Common Commensals
Common Commensals Actinobacterium meyeri Aerococcus urinaeequi Arthrobacter nicotinovorans Actinomyces Aerococcus urinaehominis Arthrobacter nitroguajacolicus Actinomyces bernardiae Aerococcus viridans Arthrobacter oryzae Actinomyces bovis Alpha‐hemolytic Streptococcus, not S pneumoniae Arthrobacter oxydans Actinomyces cardiffensis Arachnia propionica Arthrobacter pascens Actinomyces dentalis Arcanobacterium Arthrobacter polychromogenes Actinomyces dentocariosus Arcanobacterium bernardiae Arthrobacter protophormiae Actinomyces DO8 Arcanobacterium haemolyticum Arthrobacter psychrolactophilus Actinomyces europaeus Arcanobacterium pluranimalium Arthrobacter psychrophenolicus Actinomyces funkei Arcanobacterium pyogenes Arthrobacter ramosus Actinomyces georgiae Arthrobacter Arthrobacter rhombi Actinomyces gerencseriae Arthrobacter agilis Arthrobacter roseus Actinomyces gerenseriae Arthrobacter albus Arthrobacter russicus Actinomyces graevenitzii Arthrobacter arilaitensis Arthrobacter scleromae Actinomyces hongkongensis Arthrobacter astrocyaneus Arthrobacter sulfonivorans Actinomyces israelii Arthrobacter atrocyaneus Arthrobacter sulfureus Actinomyces israelii serotype II Arthrobacter aurescens Arthrobacter uratoxydans Actinomyces meyeri Arthrobacter bergerei Arthrobacter ureafaciens Actinomyces naeslundii Arthrobacter chlorophenolicus Arthrobacter variabilis Actinomyces nasicola Arthrobacter citreus Arthrobacter viscosus Actinomyces neuii Arthrobacter creatinolyticus Arthrobacter woluwensis Actinomyces odontolyticus Arthrobacter crystallopoietes -

Klaus Et Al., 1997
UC Davis UC Davis Previously Published Works Title Growth of 48 built environment bacterial isolates on board the International Space Station (ISS). Permalink https://escholarship.org/uc/item/3sw238hb Journal PeerJ, 4(3) ISSN 2167-8359 Authors Coil, David A Neches, Russell Y Lang, Jenna M et al. Publication Date 2016 DOI 10.7717/peerj.1842 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Growth of 48 built environment bacterial isolates on board the International Space Station (ISS) David A. Coil1, Russell Y. Neches1, Jenna M. Lang1, Wendy E. Brown1,2, Mark Severance2,3, Darlene Cavalier2,3 and Jonathan A. Eisen1,4,5 1 Genome Center, University of California, Davis, CA, United States 2 Science Cheerleader, Philadelphia, PA, United States 3 SciStarter.com, Philadelphia, PA, United States 4 Department of Medical Microbiology and Immunology, University of California, Davis, CA, United States 5 Department of Evolution and Ecology, University of California, Davis, CA, United States ABSTRACT Background. While significant attention has been paid to the potential risk of pathogenic microbes aboard crewed spacecraft, the non-pathogenic microbes in these habitats have received less consideration. Preliminary work has demonstrated that the interior of the International Space Station (ISS) has a microbial community resembling those of built environments on Earth. Here we report the results of sending 48 bacterial strains, collected from built environments on Earth, for a growth experiment on the ISS. This project was a component of Project MERCCURI (Microbial Ecology Research Combining Citizen and University Researchers on ISS). Results. Of the 48 strains sent to the ISS, 45 of them showed similar growth in space and on Earth using a relative growth measurement adapted for microgravity. -

Use of the Diagnostic Bacteriology Laboratory: a Practical Review for the Clinician
148 Postgrad Med J 2001;77:148–156 REVIEWS Postgrad Med J: first published as 10.1136/pmj.77.905.148 on 1 March 2001. Downloaded from Use of the diagnostic bacteriology laboratory: a practical review for the clinician W J Steinbach, A K Shetty Lucile Salter Packard Children’s Hospital at EVective utilisation and understanding of the Stanford, Stanford Box 1: Gram stain technique University School of clinical bacteriology laboratory can greatly aid Medicine, 725 Welch in the diagnosis of infectious diseases. Al- (1) Air dry specimen and fix with Road, Palo Alto, though described more than a century ago, the methanol or heat. California, USA 94304, Gram stain remains the most frequently used (2) Add crystal violet stain. USA rapid diagnostic test, and in conjunction with W J Steinbach various biochemical tests is the cornerstone of (3) Rinse with water to wash unbound A K Shetty the clinical laboratory. First described by Dan- dye, add mordant (for example, iodine: 12 potassium iodide). Correspondence to: ish pathologist Christian Gram in 1884 and Dr Steinbach later slightly modified, the Gram stain easily (4) After waiting 30–60 seconds, rinse with [email protected] divides bacteria into two groups, Gram positive water. Submitted 27 March 2000 and Gram negative, on the basis of their cell (5) Add decolorising solvent (ethanol or Accepted 5 June 2000 wall and cell membrane permeability to acetone) to remove unbound dye. Growth on artificial medium Obligate intracellular (6) Counterstain with safranin. Chlamydia Legionella Gram positive bacteria stain blue Coxiella Ehrlichia Rickettsia (retained crystal violet). -

Bacillus Pumilus Group Comparative Genomics: Toward Pangenome Features, Diversity, and Marine Environmental Adaptation
fmicb-12-571212 May 7, 2021 Time: 11:31 # 1 ORIGINAL RESEARCH published: 07 May 2021 doi: 10.3389/fmicb.2021.571212 Bacillus pumilus Group Comparative Genomics: Toward Pangenome Features, Diversity, and Marine Environmental Adaptation Xiaoteng Fu1,2,3, Linfeng Gong1,2,3, Yang Liu5, Qiliang Lai1,2,3, Guangyu Li1,2,3 and Zongze Shao1,2,3,4* 1 Key Laboratory of Marine Genetic Resources, Third Institute of Oceanography, Ministry of Natural Resources, Xiamen, China, 2 State Key Laboratory Breeding Base of Marine Genetic Resources, Xiamen, China, 3 Key Laboratory of Marine Genetic Resources of Fujian Province, Xiamen, China, 4 Southern Marine Science and Engineering Guangdong Laboratory, Zhuhai, China, 5 State Key Laboratory of Applied Microbiology Southern China, Guangdong Provincial Key Laboratory of Microbial Culture Collection and Application, Guangdong Open Laboratory of Applied Microbiology, Guangdong Microbial Culture Collection Center (GDMCC), Guangdong Institute of Microbiology, Guangdong Academy of Sciences, Guangzhou, China Edited by: John R. Battista, Background: Members of the Bacillus pumilus group (abbreviated as the Bp group) are Louisiana State University, United States quite diverse and ubiquitous in marine environments, but little is known about correlation Reviewed by: with their terrestrial counterparts. In this study, 16 marine strains that we had isolated Martín Espariz, before were sequenced and comparative genome analyses were performed with a Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), total of 52 Bp group strains. The analyses included 20 marine isolates (which included Instituto de Biología Molecular y the 16 new strains) and 32 terrestrial isolates, and their evolutionary relationships, Celular de Rosario (IBR), Argentina differentiation, and environmental adaptation. -
Bacillus Cereus
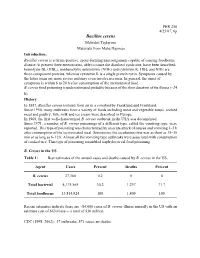
PHR 250 4/25/07, 6p Bacillus cereus Mehrdad Tajkarimi Materials from Maha Hajmeer Introduction: Bacillus cereus is a Gram-positive, spore-forming microorganism capable of causing foodborne disease At present three enterotoxins, able to cause the diarrheal syndrome, have been described: hemolysin BL (HBL), nonhemolytic enterotoxin (NHE) and cytotoxin K. HBL and NHE are three-component proteins, whereas cytotoxin K is a single protein toxin. Symptoms caused by the latter toxin are more severe and may even involve necrosis. In general, the onset of symptoms is within 6 to 24 h after consumption of the incriminated food. B. cereus food poisoning is underestimated probably because of the short duration of the illness (~24 h). History In 1887, Bacillus cereus isolated from air in a cowshed by Frankland and Frankland. Since 1950, many outbreaks from a variety of foods including meat and vegetable soups, cooked meat and poultry, fish, milk and ice cream were described in Europe. In 1969, the first well-characterized B. cereus outbreak in the USA was documented. Since 1971, a number of B. cereus poisonings of a different type, called the vomiting type, were reported. This type of poisoning was characterized by an acute attack of nausea and vomiting 1–5 h after consumption of the incriminated meal. Sometimes, the incubation time was as short as 15–30 min or as long as 6–12 h. Almost all the vomiting type outbreaks were associated with consumption of cooked rice. This type of poisoning resembled staphylococcal food poisoning. B. Cereus in the US Table 1: Best estimates of the annual cases and deaths caused by B. -

(BCID) Results Are “Not Detected”
Interpretation of Positive Blood Cultures When PCR Blood Culture Identification (BCID) Results are “Not Detected” Nebraska Medicine currently uses a multi-plex PCR-based blood culture identification (BCID) system that is able to identify 19 potential pathogens growing in blood culture. BCID generally detects over 90% of the most common causative agents in bloodstream infections; however, when microbes not included on the panel are present in a blood culture, it returns a result of “Not Detected.” This document aims to provide guidance in these scenarios supported by data collected at Nebraska Medicine from January 2018 to August 2019. Table 1: Recommendations for treatment of patients with blood cultures growing organisms not detected on BCID Gram Stain/Preliminary Likely Organism (% total BCID negative)* Recommended Treatment Culture Result Gram-positive: Aerobe Micrococcus sp. (18.1%) (most can also grow in Coagulase-negative Staphylococcus (9.3%) None anaerobic bottles) Diphtheroids (7%) None Peptostreptococcus sp. (4.4%) If therapy is desired: Anaerobe bottle only Lactobacillus sp. (2.6%) Metronidazole 500 mg PO q8h Clostridium sp. (2.6%) OR Penicillin G 4 million units IV q4h Gram-negative: Aerobe Acinetobacter sp. (1.8%) (most can also grow in Stenotrophomonas maltophilia (1.6%) Levofloxacin 750 mg IV/PO q24h anaerobic bottles) Pseudomonas fluorescens-putida group (1%) Bacteroides fragilis group (9.3%) Anaerobe bottle only Metronidazole 500 mg IV/PO q8h Fusobacterium sp. (4.7%) *A full list of isolated organisms can be found below in Table 2 Orange text = Cocci, Blue text = Bacilli (rods) Gram-Positives When BCID results as “Not Detected” but there is microbial growth, the organism is most frequently gram-positive (71%).