4. Calibration of Miocene Nannofossil Events to Orbitally Tuned Cyclostratigraphies from Ceara Rise1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Available Generic Names for Trilobites
AVAILABLE GENERIC NAMES FOR TRILOBITES P.A. JELL AND J.M. ADRAIN Jell, P.A. & Adrain, J.M. 30 8 2002: Available generic names for trilobites. Memoirs of the Queensland Museum 48(2): 331-553. Brisbane. ISSN0079-8835. Aconsolidated list of available generic names introduced since the beginning of the binomial nomenclature system for trilobites is presented for the first time. Each entry is accompanied by the author and date of availability, by the name of the type species, by a lithostratigraphic or biostratigraphic and geographic reference for the type species, by a family assignment and by an age indication of the type species at the Period level (e.g. MCAM, LDEV). A second listing of these names is taxonomically arranged in families with the families listed alphabetically, higher level classification being outside the scope of this work. We also provide a list of names that have apparently been applied to trilobites but which remain nomina nuda within the ICZN definition. Peter A. Jell, Queensland Museum, PO Box 3300, South Brisbane, Queensland 4101, Australia; Jonathan M. Adrain, Department of Geoscience, 121 Trowbridge Hall, Univ- ersity of Iowa, Iowa City, Iowa 52242, USA; 1 August 2002. p Trilobites, generic names, checklist. Trilobite fossils attracted the attention of could find. This list was copied on an early spirit humans in different parts of the world from the stencil machine to some 20 or more trilobite very beginning, probably even prehistoric times. workers around the world, principally those who In the 1700s various European natural historians would author the 1959 Treatise edition. Weller began systematic study of living and fossil also drew on this compilation for his Presidential organisms including trilobites. -

Volume 144 Index
Index to Volume 144 INDEX TO VOLUME 144 This index provides coverage for both the Initial Reports and Scientific Results portions of Volume 144 of the Proceedings of the Ocean Drilling Program. Refer- ences to page numbers in the Initial Reports are preceded by “A” with a colon (A:), and to those in the Scientific Results (this book), by “B” with a colon (B:). In addi- tion, reference to material on CD-ROM is shown as “bp:CD-ROM.” The index was prepared by Earth Systems, under subcontract to the Ocean Drill- ing Program. The index contains two hierarchies of entries: (1) a main entry, defined as a keyword or concept followed by a reference to the page on which that word or concept appears, and (2) a subentry, defined as an elaboration on the main entry fol- lowed by a page reference. The index is presented in two parts: (1) a Subject Index and (2) a Taxonomic Index. Both parts cover text, figures, and tables but not core-description forms (“barrel sheets”), core photographs, smear-slide data, or thin-section descriptions; these are given in the Initial Reports. Also excluded from the index are biblio- graphic references, names of individuals, and routine front and back matter. The Subject Index follows a standard format. Geographic, geologic, and other terms are referenced only if they are subjects of discussion. This index also includes broad fossil groups such as nannofossils and radiolarians. A site chapter in the Ini- tial Reports is considered the principal reference for that site and is indicated on the first line of the site’s listing in the index. -

Applications of Calcareous Nannofossils and Stable Isotopes To
Florida State University Libraries Electronic Theses, Treatises and Dissertations The Graduate School 2007 Applications of Calcareous Nannofossils and Stable Isotopes to Cenozoic Paleoceanography: Examples from the Eastern Equatorial Pacific, Western Equatorial Atlantic and Southern Indian Oceans Shijun Jiang Follow this and additional works at the FSU Digital Library. For more information, please contact [email protected] THE FLORIDA STATE UNIVERSITY COLLEGE OF ARTS AND SCIENCES APPLICATIONS OF CALCAREOUS NANNOFOSSILS AND STABLE ISOTOPES TO CENOZOIC PALEOCEANOGRAPHY: EXAMPLES FROM THE EASTERN EQUATORIAL PACIFIC, WESTERN EQUATORIAL ATLANTIC AND SOUTHERN INDIAN OCEANS By SHIJUN JIANG A dissertation submitted to the Department of Geological Sciences in partial fulfillment of the requirements for the degree of Doctor of Philosophy Degree Awarded: Fall Semester, 2007 The members of the Committee approve the Dissertation of Shijun Jiang defended on July 13, 2007. ____________________________________ Sherwood W. Wise, Jr. Professor Directing Dissertation ____________________________________ Richard L. Iverson Outside Committee Member ____________________________________ Anthony J. Arnold Committee Member ____________________________________ Joseph F. Donoghue Committee Member ____________________________________ Yang Wang Committee Member The Office of Graduate Studies has verified and approved the above named committee members. ii To Shuiqing and Jenny iii ACKNOWLEDGEMENTS First of all, I would like to thank my mentor Dr. Sherwood W. Wise, Jr., who constantly encouraged and supported me with his enthusiasm, reliance, guidance and, most of all, patience throughout my Ph.D adventure. He also opened a door into my knowledge of the paleo world, generously shared his time and his wealth of knowledge, patiently guided me through a western educational system totally different from my own background, and has successfully fostered my interest and enthusiasm in teaching. -

Cenozoic-Mesozoic-Paleozoic Integrated Stratigraphy and User- Generated Time Scale Graphics and Charts
GeoArabia, Vol. 11, No. 3, 2006 Gulf PetroLink, Bahrain LETTER TO THE EDITOR hank you again for another outstanding issue of GeoArabia (vol. 11, no. 1, 2006). There is Tabsolutely no doubt that GeoArabia is the best stratigraphic journal in the world. This is for the simple reason that the geoscience described in this journal directly uses and directly refers to Stratigraphy in all its aspects and in a very comprehensive manner. The published papers are outstanding and serve the upstream industry, as well as scientific research and educational institutions. The International Commission on Stratigraphy (ICS) is presently working on improving the highly popular program Time Scale Creator (TS-Creator©) that can be downloaded freely from the ICS website. This data base is coordinated by Jim Ogg and the program was developed by Adam Lugowski. It provides large bio-, magneto-, chemo- and chrono-sequence stratigraphic charts for specific basins and specific time intervals. The ICS would like to extend this work to the Middle East basins by working closely with geoscientists from your region. In this regard we thank GeoArabia for explaining the studies conducted by the ICS to the Middle East geoscience community by publishing the feature on the ICS (vol. 11, no. 1, p. 159–160) and the following article. We look forward to hearing back from interested Middle East stratigraphers. Felix Gradstein Chair, International Commission on Stratigraphy TS-Creator© - Chronostratigraphic data base and visualisation: Cenozoic-Mesozoic-Paleozoic integrated stratigraphy and user- generated time scale graphics and charts Felix Gradstein and James Ogg Summary The International Commission on Stratigraphy (ICS) has produced an electronic version of the international standard Cenozoic-Mesozoic-Paleozoic bio-magneto-sequence, time-scale charts. -

Cenozoic Fossil Mollusks from Western Pacific Islands; Gastropods (Eratoidae Through Harpidae)
Cenozoic Fossil Mollusks From Western Pacific Islands; Gastropods (Eratoidae Through Harpidae) GEOLOGICAL SURVEY PROFESSIONAL PAPER 533 Cenozoic Fossil Mollusks From Western Pacific Islands; Gastropods (Eratoidae Through Harpidae) By HARRY S. LADD GEOLOGICAL SURVEY PROFESSIONAL PAPER 533 Descriptions or citations of 195 representatives of 21 gastropod families from 7 island groups UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1977 UNITED STATES DEPARTMENT OF THE INTERIOR CECIL D. ANDRUS, Secretary GEOLOGICAL SURVEY V. E. McKelvey, Director Library of Congress Cataloging in Publication Data Ladd, Harry Stephen, 1899- Cenozoic fossil mollusks from western Pacific islands. (Geological Survey professional paper ; 533) Bibliography: p. Supt. of Docs, no.: I 19.16:533 1. Gastropoda, Fossil. 2. Paleontology Cenozoic. 3. Paleontology Islands of the Pacific. I. Title. II. Series: United States. Geological Survey. Professional paper ; 533. QE75.P9 no. 533 [QE808] 557.3'08s [564'.3'091646] 75-619274 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 Stock Number 024-001-02975-8 CONTENTS Page Page Abstract _ _ _ _ ___ _ _ 1 Paleontology Continued Introduction ____ _ _ __ 1 Systematic paleontology Continued 1 Families covered in the present paper Continued Stratigraphy and correlation _ _ _ q Cymatiidae 33 6 35 Tonnidae __ _______ 36 6 Ficidae _ - _ _ _ _ ___ 37 Fiji _ __ __ __ _____ __ ____ __ _ 6 37 New Hebrides 7 Thaididae __ _ _ _ _ _ 39 14 41 14 Columbellidae - 44 14 Buccinidae _ - - 49 51 (1966, 1972) 14 Nassariidae _ - 51 "P1 ?} TYllllPQ. -

14. Oligocene to Recent Calcareous Nannoplankton from the Philippine Sea, Deep Sea Drilling Project Leg 59
14. OLIGOCENE TO RECENT CALCAREOUS NANNOPLANKTON FROM THE PHILIPPINE SEA, DEEP SEA DRILLING PROJECT LEG 59 Erlend Martini, Geologisch-Palàontologisches Institut der Universitàt, Frankfurt am Main, Germany INTRODUCTION substitute species. Its last occurrence marks the top of Zone NP 25 in Leg 59. During Leg 59 of the Deep Sea Drilling Project, five A different zonation, mainly based on Bukry (1971a, sites (447 to 451) were occupied and seven holes drilled 1973), was used during Leg 31 in the Western Philippine between Okinawa and Guam in the Philippine Sea (Fig. Sea and during Leg 60 at the eastern transect of the 1). All holes except Hole 447 yielded common calcare- Philippine Sea. For better comparison of results both ous nannoplankton at certain intervals cored. Nanno- zonations and their correlation are shown in Figure 2. plankton assemblages and their age assignments will be The zonations differ in some parts of the tabulated time discussed for each site, and fossil lists for selected interval but are otherwise very similar because 20 boun- samples from Holes 447A, 448, 450, and 451 are pre- daries are identical in both zonations. There is also sented in Tables 1 to 4, covering the middle Oligocene to general agreement on the age of some major boun- the Quaternary. daries, indicated by an asterisk in Figure 2. A few remarks, however, seem necessary to avoid misinter- CALCAREOUS NANNOPLANKTON ZONATION pretation, especially in the Oligocene, where some con- For the Tertiary and Quaternary, I have used the fusion may arise because the same zonal names are used standard calcareous nannonplankton zonation (Mar- for different time intervals. -

Paleontology of the Upper Eocene to Quaternary Postimpact Section in the USGS-NASA Langley Core, Hampton, Virginia
Paleontology of the Upper Eocene to Quaternary Postimpact Section in the USGS-NASA Langley Core, Hampton, Virginia By Lucy E. Edwards, John A. Barron, David Bukry, Laurel M. Bybell, Thomas M. Cronin, C. Wylie Poag, Robert E. Weems, and G. Lynn Wingard Chapter H of Studies of the Chesapeake Bay Impact Structure— The USGS-NASA Langley Corehole, Hampton, Virginia, and Related Coreholes and Geophysical Surveys Edited by J. Wright Horton, Jr., David S. Powars, and Gregory S. Gohn Prepared in cooperation with the Hampton Roads Planning District Commission, Virginia Department of Environmental Quality, and National Aeronautics and Space Administration Langley Research Center Professional Paper 1688 U.S. Department of the Interior U.S. Geological Survey iii Contents Abstract . .H1 Introduction . 1 Previous Work and Zonations Used . 3 Lithostratigraphy of Postimpact Deposits in the USGS-NASA Langley Corehole . 7 Methods . 8 Paleontology . 9 Chickahominy Formation . 9 Drummonds Corner Beds . 17 Old Church Formation . 19 Calvert Formation . 20 Newport News Beds . 20 Plum Point Member . 20 Calvert Beach Member . 21 St. Marys Formation . 27 Eastover Formation . 28 Yorktown Formation . 29 Tabb Formation . 31 Discussion . 31 Summary and Conclusions . 31 Acknowledgments . 33 References Cited . 34 Appendix H1. Full Taxonomic Citations for Taxa Mentioned in Chapter H . 39 Appendix H2. Useful Cenozoic Calcareous Nannofossil Datums . 46 Plates [Plates follow appendix H2] H1–H9. Fossils from the USGS-NASA Langley core, Hampton, Va.: H1. Dinoflagellate cysts from the Chickahominy Formation H2. Dinoflagellate cysts from the Chickahominy Formation H3. Dinoflagellate cysts from the Chickahominy Formation, Drummonds Corner beds, and Old Church Formation H4. Dinoflagellate cysts from the Old Church and Calvert Formations H5. -

Coccolithophores
Coccolithophores From Molecular Processes to Global Impact Bearbeitet von Hans R Thierstein, Jeremy R Young 1. Auflage 2004. Buch. xiii, 565 S. Hardcover ISBN 978 3 540 21928 6 Format (B x L): 21 x 29,7 cm Gewicht: 1129 g Weitere Fachgebiete > Chemie, Biowissenschaften, Agrarwissenschaften > Botanik > Phykologie Zu Inhaltsverzeichnis schnell und portofrei erhältlich bei Die Online-Fachbuchhandlung beck-shop.de ist spezialisiert auf Fachbücher, insbesondere Recht, Steuern und Wirtschaft. Im Sortiment finden Sie alle Medien (Bücher, Zeitschriften, CDs, eBooks, etc.) aller Verlage. Ergänzt wird das Programm durch Services wie Neuerscheinungsdienst oder Zusammenstellungen von Büchern zu Sonderpreisen. Der Shop führt mehr als 8 Millionen Produkte. What is new in coccolithophore biology? Chantal BILLARD1 and Isao INOUYE2 1 Laboratoire de Biologie et Biotechnologies Marines, Université de Caen, F-14032 Caen, France. [email protected] 2 Institute of Biological Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, 305- 8572 Ibaraki, Japan. [email protected] Summary Knowledge of the biology of coccolithophores has progressed considerably in re- cent years thanks to culture studies and meticulous observations of coccospheres in wild samples. It has been confirmed that holococcolithophores and other "anomalous" coccolithophores are not autonomous but stages in the life cycle of oceanic heterococcolithophores. The existence of such heteromorphic life cycles linking former "species" has far reaching consequences on the taxonomy and no- menclature of coccolithophores and should foster research on the environmental factors triggering phase changes. The cytological characteristics of coccolithopho- res are reviewed in detail with special attention to the cell covering, coccolitho- genesis and the specificity of appendages in this group. -
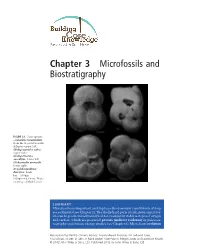
Microfossils and Biostratigraphy
Chapter 3 Microfossils and Biostratigraphy FIGURE 3.1. Four species of planktic foraminifers from the tropical western Atlantic: upper left: Globigerinoides ruber, upper right: Globigerinoides sacculifer, lower left: Globorotalia menardii, lower right: Neogloboquadrina dutertrei. Scale bar = 100 µm (100 µm = 0.1 mm). Photo courtesy of Mark Leckie. SUMMARY Microfossils are important, and in places the dominant constituents of deep sea sediments (see Chapter 2). The shells/hard parts of calcareous microfos- sils can be geochemically analyzed, for example by stable isotopes of oxygen and carbon, which are powerful proxies (indirect evidence) in paleocea- nography and climate change studies (see Chapter 6). Microfossil evolution Reconstructing Earth’s Climate History: Inquiry-Based Exercises for Lab and Class, First Edition. Kristen St John, R Mark Leckie, Kate Pound, Megan Jones and Lawrence Krissek. © 2012 John Wiley & Sons, Ltd. Published 2012 by John Wiley & Sons, Ltd. NAME has provided a rich archive for establishing relative time in marine sedi- mentary sequences. In this chapter you will gain experience using micro- fossil distributions in deep-sea cores to apply a biostratigraphic zonation and interpret relative age, correlate from one region of the world ocean to another, and calculate rates of sediment accumulation. In Part 3.1, you will learn about the major types of marine microfossils and their habitats; you will consider their role in primary productivity in the world oceans, their geologic record and diversity through time, as well as the relationship between microfossil diversity and sea level changes through time. In Part 3.2, you will explore microfossil distribution in a deep-sea core and consider microfossils as age indicators. -

Reproductive Biology of Plants
K21132 6000 Broken Sound Parkway, NW Suite 300, Boca Raton, FL 33487 711 Third Avenue New York, NY 10017 an informa business 2 Park Square, Milton Park A SCIENCE PUBLISHERS BOOK www.crcpress.com Abingdon, Oxon OX14 4RN, UK About the pagination of this eBook Due to the unique page numbering scheme of this book, the electronic pagination of the eBook does not match the pagination of the printed version. To navigate the text, please use the electronic Table of Contents that appears alongside the eBook or the Search function. For citation purposes, use the page numbers that appear in the text. Reproductive Biology of Plants Reproductive Biology of Plants Editors K.G. Ramawat Former Professor & Head Botany Department, M.L. Sukhadia University Udaipur, India Jean-Michel Mérillon Université de Bordeaux Institut des Sciences de la Vigne et du Vin Villenave d’Ornon, France K.R. Shivanna Former Professor & Head Botany Department, University of Delhi Delhi, India p, A SCIENCE PUBLISHERS BOOK GL--Prelims with new title page.indd ii 4/25/2012 9:52:40 AM CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2014 by Taylor & Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Version Date: 20140117 International Standard Book Number-13: 978-1-4822-0133-8 (eBook - PDF) This book contains information obtained from authentic and highly regarded sources. Reasonable efforts have been made to publish reliable data and information, but the author and publisher cannot assume responsibility for the validity of all materials or the consequences of their use. -

Deep Sea Drilling Project Initial Reports Volume 13
13. TYRRHENIAN RISE - SITE 132 The Shipboard Scientific Party1 SITE DATA convinced as to such permanence, other workers have envisioned the creation of new areas of young crust by the Occupied: September 24-26, 1970. opening of sphenochasms and the. drifting apart of Position: On a relatively smooth rise approximately 60 continents following the break up of Pangaea (Carey, 1958; kilometers west and about 800 meters above the level of Vogt et al., 1971). The proposed age of the extensional the central bathyal plain. phases in the western Mediterranean, during which simatic Latitude: 40° 15.7θ'N crust was suspected to have been accreted, spans a broad Longitude: 11° 26.47'E range of time from the Lower Jurassic (Smith, 1971) to the Water Depth: 2835 meters. late Eocene-Oligocene (Ryan et al, 1971; Le Pichon et al, Cores Taken: Twenty-seven. 1971), and even into the Miocene (Argand, 1922; Stanley Total Penetration: 223 meters. andMutti, 1968). There are still others who argue that the present bathyal Deepest Unit Recovered: Solid gypsum rock of Upper areas of the Tyrrhenian Sea came into existence following Miocene (Messinian) age displaying undulating lami- the collapse of a craton (e.g., Merla, 1951; Klemme, 1958; nations characteristic of algal stromatolites. Maxwell, 1959; Aubouin, 1965; Pannekoek, 1969; Van MAIN RESULTS Bemmelen, 1969; Morelli, 1970; Heezen et al, 1971). As to when the oceanization occurred, a recent paper of Selli and A continuous sequence of Pleistocene and Pliocene Fabbri (1971) reports on fossil assemblages found in fossiliferous pelagic ooze of a deep-water (bathyal) facies "littoral" conglomerates recovered in dredge samples from was found directly overlying Late Miocene evaporites which the Stromboli and Oresi Canyons and the Baronie include barren pyritic, dolomitic, and gypsiferous marls, Seamount (Figure 1), and concludes that the foundering and indurated gypsum rock. -

Redalyc.The Pliocene Mediterranean Infilling of the Messinian Erosional
Geologica Acta: an international earth science journal ISSN: 1695-6133 [email protected] Universitat de Barcelona España LANCIS, C.; TENT-MANCLÚS, J.E.; FLORES, J.A.; SORIA, J.M. The Pliocene Mediterranean infilling of the Messinian Erosional Surface: New biostratigraphic data based on calcareous nannofossils (Bajo Segura Basin, SE Spain) Geologica Acta: an international earth science journal, vol. 13, núm. 3, septiembre, 2015, pp. 211-228 Universitat de Barcelona Barcelona, España Available in: http://www.redalyc.org/articulo.oa?id=50541392004 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Geologica Acta, Vol. 13, Nº 3, September 2015, 211-228 DOI: 10.1344/GeologicaActa2015.13.3.4 The Pliocene Mediterranean infilling of the Messinian Erosional Surface: New biostratigraphic data based on calcareous nannofossils (Bajo Segura Basin, SE Spain) C. LANCIS1 J.E. TENT-MANCLÚS1* J.A. FLORES2 J.M. SORIA1 1Departamento de Ciencias de la Tierra y del Medio Ambiente, Universidad de Alicante Apto. 99. 03080 San Vicente del Raspeig. Alicante, Spain. Lancis E-mail: [email protected], Tent-Manclús E-mail: [email protected], Soria E-mail: [email protected] Fax: (+ 34) 965909862 2Departamento de Geología, Universidad de Salamanca Plaza de la Merced s/n, 37008-Salamanca, Spain. E-mail: [email protected] Fax: (+ 34) 923294514 *Corresponding author ABS TRACT The Bajo Segura Basin (eastern Betic Cordillera) is a Mediterranean marginal basin where the Messinian Erosional Surface (MES), formed during the Messinian Salinity Crisis sea-level fall, is well developed.